Prevalence and clinical characteristics of perinatal chronic lung disease by infant gestational age
- PMID: 32474477
- PMCID: PMC7990434
- DOI: 10.3233/NPM-200412
Prevalence and clinical characteristics of perinatal chronic lung disease by infant gestational age
Abstract
Background: Children with perinatal chronic lung disease (CLD) are at elevated risk for severe respiratory syncytial virus (RSV) disease in the first two years of life. The American Academy of Pediatrics policy does not recommend RSV immunoprophylaxis for infants with CLD born at ≥32 weeks' gestational age (wGA). The objective of this study was to describe the number and clinical characteristics of US infants in this population.
Methods: Birth hospitalization data from the Kids' Inpatient Database were utilized to estimate the prevalence of CLD (International Classification of Diseases, Ninth Revision [ICD-9] = 770.7) in 2003-2012 overall and by gestational age (ICD-9 = 765.21-765.29). CLD birth hospitalizations were evaluated by length of stay, costs, ventilatory support, and inpatient mortality.
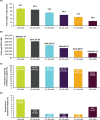
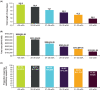
Results: A total of 33,537 infants were diagnosed with CLD, representing 0.2% of US births; 79% had wGA coded in the database. Among infants with CLD with wGA, 3.5% were born at >32 wGA, representing 7 of every 100,000 US births, or approximately 300 infants annually. Across all wGA categories, birth hospitalization length of stay and costs were elevated, and mechanical ventilation use ranged from 73% to 97%. All-cause inpatient mortality was highest among those <27 wGA and >32 wGA.
Conclusions: Approximately 300 infants born at >32 wGA are diagnosed with CLD annually in the United States. The all-cause perinatal mortality rate is high in this population. The rationale for excluding this small but high-risk group of infants from the recommendations for RSV immunoprophylaxis is unclear.
Keywords: Bronchopulmonary dysplasia; immunoprophylaxis; respiratory syncytial virus.
Conflict of interest statement
This research was funded by AstraZeneca, the manufacturer of palivizumab. The sponsor was involved in the design and conduct of the study and approval of the manuscript. Decision rights about content belonged collectively to the authors and not to the sponsor. No substantive changes were made to the manuscript as a result of the company review. KM is on the speakers bureau for AstraZeneca. XJ provided consultant/research support for AstraZeneca. CSA is an employee of AstraZeneca.
Figures

References
-
- Romagnoli C, Zecca E, Tortorolo L, Vento G, Tortorolo G. A scoring system to predict the evolution of respiratory distress syndrome into chronic lung disease in preterm infants. Intensive Care Med. 1998;24(5):476–80. - PubMed
-
- Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: Selection criteria for clinical trials. Pediatrics. 1990;86(5):728–36. - PubMed
-
- IMpact RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531–7. - PubMed
-
- Healthcare Cost and Utilization Project (HCUP) [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; [cited 2019 Dec 26]. Available from: www.hcup-us.ahrq.gov/kidoverview.jsp.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

