Oral S-1 with 24-h Infusion of Irinotecan plus Bevacizumab versus FOLFIRI plus Bevacizumab as First-Line Chemotherapy for Metastatic Colorectal Cancer: An Open-Label Randomized Phase II Trial
- PMID: 32474564
- PMCID: PMC7592907
- DOI: 10.1159/000507293
Oral S-1 with 24-h Infusion of Irinotecan plus Bevacizumab versus FOLFIRI plus Bevacizumab as First-Line Chemotherapy for Metastatic Colorectal Cancer: An Open-Label Randomized Phase II Trial
Abstract
Background: FOLFIRI plus bevacizumab have been widely used as first-line treatment for metastatic colorectal cancer (mCRC). Pharmacokinetics and pharmacodynamics suggested a low dose of irinotecan given as a long-term infusion is expected to enhance antitumor activity. We conducted a randomized phase II study to compare oral S-1 with a 24-h infusion of irinotecan plus bevacizumab versus FOLFIRI plus bevacizumab.
Methods: The subjects comprised 120 chemotherapy-naïve patients with mCRC. The study group received a 24-h infusion of irinotecan at a dose of 125 mg/m2 on days 1 and 15, combined with oral S-1 80 mg/m2 on days 1-14 (24h-SIRI/B). The FOLFIRI/B group received irinotecan at a dose of 150 mg/m2, 5-fluorouracil given at a dose of 400 mg/m2 as a bolus injection and at a dose of 2,400 mg/m2 as a 46-h infusion, and 200 mg/m2 leucovorin on days 1 and 15. Bevacizumab was given at a dose of 5.0 mg/kg on days 1 and 15 in both groups. Treatment was repeated every 4 weeks. The primary endpoint was 1-year progression-free survival (PFS). Secondary endpoints were PFS, response rates (RR), overall survival (OS), and adverse events (AEs).
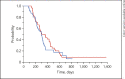
Results: From December 2013 through January 2018, 120 patients were randomly assigned, 61 patients to the 24h-SIRI/B and 59 patients to the FOLFIRI/B. The median follow-up period was 22.8 months. The 1-year PFS rate was 43.14% in the 24h-SIRI/B arm and 19.15% in the FOLFIRI/B arm (HR = 0.312 [95%CI 0.13-0.78], p = 0.01). The median PFS was 10.2 months (95%CI 8.8-14.3) and 10.0 months (95%CI 7.4-11.0), and the median OS was 29.7 months (95%CI 22.9-43.9) and 28.8 months (95%CI 18.4-ND), respectively (p = 0.3758, p = 0.8234). The overall RR was 86.3 and 61.7%, respectively (p = 0.0053). AEs were similar.
Conclusions: Our results show that the 24h-SIRI/B regimen is an effective and reasonably well-tolerated regimen for the first-line treatment of mCRC.
Keywords: 24-h infusion; Bevacizumab; Colorectal cancer; FOLFIRI; Irinotecan; Phase II study; S-1.
© 2020 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
All authors have no potential conflicts of interest to declare.
Figures
References
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014 Sep;25((Suppl 3)):iii1–9. - PubMed
-
- National Comprehensive Cancer Network (NCCN) Colon Cancer [Internet] [cited 2018 Oct 19]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
-
- Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Ann Oncol. 2016 Aug;27((8)):1539–46. - PubMed
-
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009 Jul 1;27((19)):3109–16. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical