Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50
- PMID: 32474768
- PMCID: PMC11027629
- DOI: 10.1007/s00262-020-02613-9
Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50
Abstract
Background: Single-agent pembrolizumab represents the standard first-line option for metastatic non-small-cell lung cancer (NSCLC) patients with a PD-L1 (programmed death-ligand 1) expression of ≥ 50%.
Methods: We conducted a multicenter retrospective study aimed at evaluating the clinicopathologic correlates of pembrolizumab effectiveness in patients with treatment-naïve NSCLC and a PD-L1 expression of ≥ 50%.
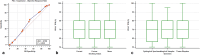
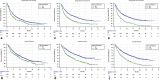
Results: One thousand and twenty-six consecutive patients were included. The objective response rate (ORR) was 44.5% (95% CI 40.2-49.1), while the median progression free survival (PFS) and overall survival (OS) were 7.9 months (95% CI 6.9-9.5; 599 events) and 17.2 months (95% CI 15.3-22.3; 598 censored patients), respectively. ECOG-PS ≥ 2 (p < 0.0001) and bone metastases (p = 0.0003) were confirmed to be independent predictors of a worse ORR. Former smokers (p = 0.0002), but not current smokers (p = 0.0532) were confirmed to have a significantly prolonged PFS compared to never smokers at multivariate analysis. ECOG-PS (p < 0.0001), bone metastases (p < 0.0001) and liver metastases (p < 0.0001) were also confirmed to be independent predictors of a worse PFS. Previous palliative RT was significantly related to a shortened OS (p = 0.0104), while previous non-palliative RT was significantly related to a prolonged OS (p = 0.0033). Former smokers (p = 0.0131), but not current smokers (p = 0.3433) were confirmed to have a significantly prolonged OS compared to never smokers. ECOG-PS (p < 0.0001), bone metastases (p < 0.0001) and liver metastases (p < 0.0001) were also confirmed to be independent predictors of a shortened OS. A PD-L1 expression of ≥ 90%, as assessed by recursive partitioning, was associated with significantly higher ORR (p = 0.0204), and longer and OS (p = 0.0346) at multivariable analysis.
Conclusion: Pembrolizumab was effective in a large cohort of NSCLC patients treated outside of clinical trials. Questions regarding the effectiveness in clinical subgroups, such as patients with poorer PS and with liver/bone metastases, still remain to be addressed. We confirmed that the absence of tobacco exposure, and the presence of bone and liver metastasis are associated with worse clinical outcomes to pembrolizumab. Increasing levels of PD-L1 expression may help identifying a subset of patients who derive a greater benefit from pembrolizumab monotherapy.
Keywords: Bone metastases; Liver metastases; PD-L1; Performance status; Radiotherapy; Smoking status.
Conflict of interest statement
Dr. Alessio Cortellini received speaker fees and grant consultancies by Astrazeneca, MSD, BMS, Roche, Novartis, Istituto Gentili, Astellas and Ipsen. Dr. Emilio Bria received speaker and travel fees from MSD, Astra-Zeneca, Pfizer, Helsinn, Eli-Lilly, BMS, Novartis and Roche. Dr. Emilio Bria received grant consultancies by Roche and Pfizer. Dr. Marcello Tiseo received speaker fees and grant consultancies by Astrazeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda and Pierre Fabre. Dr. Alessandro Morabito received speaker fees by Astra, Roche, BMS, MSD, Boehringer, Pfizer, Takeda. Dr. Francesca Mazzoni received grant consultancies by MSD and Takeda. Dr. Raffaele Gisti received speaker fees and grant consultancies by Astrazeneca and Roche. Dr. Francesco Passiglia received grant consultancies by MSD and Astrazeneca. Dr. Paolo Bironzo received grant consultancies by Astrazeneca and Boehringer-Ingelheim. Dr. Alex Friedlaender received grant consultancies by Roche, Pfizer, Astellas and BMS. Dr. Alfredo Addeo received grant consultancies by Takeda, MSD, BMJ, Astrazeneca, Roche and Pfizer. Dr. Rita Chiari received speaker fees by BMS, MSD, Takeda, Pfizer, Roche and Astrazeneca. Dr. Carlo Genova received speaker fees/grant consultancies by Astrazeneca, BMS, Boehringer-Ingelheim, Roche and MSD.
Figures


References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. OA14.01 KEYNOTE-024 3-year survival update: pembrolizumab vs platinum-based chemotherapy for advanced non-small-cell lung cancer. J Thorac Oncol. 2019;14(10):S243. doi: 10.1016/j.jtho.2019.08.483. - DOI
-
- Tamiya M, Tamiya A, Hosoya K, et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001) Investig New Drugs. 2019;37(6):1266–1273. doi: 10.1007/s10637-019-00843-y. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

