Valorisation of pectin-rich agro-industrial residues by yeasts: potential and challenges
- PMID: 32474799
- PMCID: PMC7347521
- DOI: 10.1007/s00253-020-10697-7
Valorisation of pectin-rich agro-industrial residues by yeasts: potential and challenges
Abstract
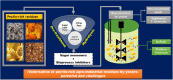
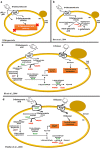
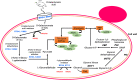
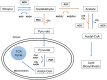
Pectin-rich agro-industrial residues are feedstocks with potential for sustainable biorefineries. They are generated in high amounts worldwide from the industrial processing of fruits and vegetables. The challenges posed to the industrial implementation of efficient bioprocesses are however manyfold and thoroughly discussed in this review paper, mainly at the biological level. The most important yeast cell factory platform for advanced biorefineries is currently Saccharomyces cerevisiae, but this yeast species cannot naturally catabolise the main sugars present in pectin-rich agro-industrial residues hydrolysates, in particular D-galacturonic acid and L-arabinose. However, there are non-Saccharomyces species (non-conventional yeasts) considered advantageous alternatives whenever they can express highly interesting metabolic pathways, natively assimilate a wider range of carbon sources or exhibit higher tolerance to relevant bioprocess-related stresses. For this reason, the interest in non-conventional yeasts for biomass-based biorefineries is gaining momentum. This review paper focuses on the valorisation of pectin-rich residues by exploring the potential of yeasts that exhibit vast metabolic versatility for the efficient use of the carbon substrates present in their hydrolysates and high robustness to cope with the multiple stresses encountered. The major challenges and the progresses made related with the isolation, selection, sugar catabolism, metabolic engineering and use of non-conventional yeasts and S. cerevisiae-derived strains for the bioconversion of pectin-rich residue hydrolysates are discussed. The reported examples of value-added products synthesised by different yeasts using pectin-rich residues are reviewed. Key Points • Review of the challenges and progresses made on the bioconversion of pectin-rich residues by yeasts. • Catabolic pathways for the main carbon sources present in pectin-rich residues hydrolysates. • Multiple stresses with potential to affect bioconversion productivity. • Yeast metabolic engineering to improve pectin-rich residues bioconversion. Graphical abstract.
Keywords: Bioconversion; Biorefinery; Circular bioeconomy; Metabolic engineering; Non-conventional yeasts; Pectin-rich agro-industrial residues.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Aksu Z, Eren AT. Carotenoids production by the yeast Rhodotorula mucilaginosa: use of agricultural wastes as a carbon source. Process Biochem. 2005;40:2985–2991. doi: 10.1016/j.procbio.2005.01.011. - DOI
-
- Anschau A (2017) Lipids from oleaginous yeasts: production and encapsulation. In: Nutrient Delivery. Elsevier, pp 749–794
-
- Babel W. The auxiliary substrate concept: from simple considerations to heuristically valuable knowledge. Eng Life Sci. 2009;9:285–290. doi: 10.1002/elsc.200900027. - DOI
-
- Balat M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag. 2011;52:858–875. doi: 10.1016/j.enconman.2010.08.013. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

