Population pharmacokinetic approach for evaluation of treosulfan and its active monoepoxide disposition in plasma and brain on the basis of a rat model
- PMID: 32474888
- PMCID: PMC7550288
- DOI: 10.1007/s43440-020-00115-0
Population pharmacokinetic approach for evaluation of treosulfan and its active monoepoxide disposition in plasma and brain on the basis of a rat model
Erratum in
-
Correction to: Population pharmacokinetic approach for evaluation of treosulfan and its active monoepoxide disposition in plasma and brain on the basis of a rat model.Pharmacol Rep. 2020 Oct;72(5):1443. doi: 10.1007/s43440-020-00144-9. Pharmacol Rep. 2020. PMID: 32785852 Free PMC article.
Abstract
Purpose: Efficacy of treosulfan, used in the treatment of marrow disorders, depends on the activity of its monoepoxy-(EBDM) and diepoxy compounds. The study aimed to describe the pharmacokinetics of treosulfan and EBDM in the rat plasma and brain by means of mixed-effects modelling.
Methods: The study had a one-animal-per-sample design and included ninty-six 10-week-old Wistar rats of both sexes. Treosulfan and EBDM concentrations in the brain and plasma were measured by an HPLC-MS/MS method. The population pharmacokinetic model was established in NONMEM software with a first-order estimation method with interaction.
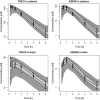
Results: One-compartment pharmacokinetic model best described changes in the concentrations of treosulfan in plasma, and EBDM concentrations in plasma and in the brain. Treosulfan concentrations in the brain followed a two-compartment model. Both treosulfan and EBDM poorly penetrated the blood-brain barrier (ratio of influx and efflux clearances through the blood-brain barrier was 0.120 and 0.317 for treosulfan and EBDM, respectively). Treosulfan plasma clearance was significantly lower in male rats than in females (0.273 L/h/kg vs 0.419 L/h/kg).
Conclusions: The developed population pharmacokinetic model is the first that allows the prediction of treosulfan and EBDM concentrations in rat plasma and brain. These results provide directions for future studies on treosulfan regarding the contribution of transport proteins or the development of a physiological-based model.
Keywords: Alkylating antineoplastic agents; Blood–brain barrier; Epoxy compounds; Population pharmacokinetics.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures



References
-
- Park S, Tretyakova N. Structural characterization of the major DNA-DNA cross-link of 1,2,3,4-diepoxybutane. Chem Res Toxicol. 2004;17:129–136. - PubMed
-
- Romański M, Girreser U, Teżyk A, Główka FK. N-7-guanine adduct of the active monoepoxide of prodrug treosulfan: first synthesis, characterization, and decomposition profile under physiological conditions. J Pharm Sci. 2018;107:2927–2937. - PubMed
-
- Shimoni A, Labopin M, Savani B, Hamladji R-M, Beelen D, Mufti G, et al. Intravenous busulfan compared with treosulfan-based conditioning for allogeneic stem cell transplantation in acute myeloid leukemia: a study on behalf of the acute leukemia working party of european society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2018;24:751–757. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
