Transcriptional activity of vitamin D receptor in human periodontal ligament cells is diminished under inflammatory conditions
- PMID: 32474936
- PMCID: PMC7891446
- DOI: 10.1002/JPER.19-0541
Transcriptional activity of vitamin D receptor in human periodontal ligament cells is diminished under inflammatory conditions
Abstract
Background: Although vitamin D3 deficiency is considered as a risk factor for periodontitis, supplementation during periodontal treatment has not been shown to be beneficial to date. Human periodontal ligament cells (hPDLCs) are regulated by vitamin D3 and play a fundamental role in periodontal tissue homeostasis and inflammatory response in periodontitis. The aim of this study is to investigate possible alterations of the vitamin D3 activity in hPDLCs under inflammatory conditions.
Methods: Cells isolated from six different donors were treated with either 1,25(OH)2 D3 (0 to 10 nM) or 25(OH)D3 (0 to 100 nM) in the presence and absence of ultrapure or standard Porphyromonas gingivalis lipopolysaccharide (PgLPS), Pam3CSK4, or interferon-γ for 48 hours. Additionally, nuclear factor (NF)-κB inhibition was performed with BAY 11-7082. The bioactivity of vitamin D in hPDLCs was assessed based on the gene expression levels of vitamin D receptor (VDR)-regulated genes osteocalcin and osteopontin. Additionally, VDR and CYP27B1 expression levels were measured.
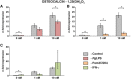
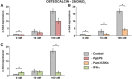
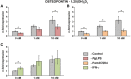
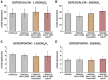
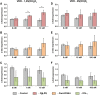
Results: The vitamin D3 -induced increase of osteocalcin and osteopontin expression was significantly decreased in the presence of standard PgLPS and Pam3CSK4, which was not observed by ultrapure PgLPS. Interferon-y had diverse effects on the response of hPDLCs to vitamin D3 metabolites. NF-kB inhibition abolished the effects of standard PgLPS and Pam3CSK4. Standard PgLPS and Pam3CSK4 increased VDR expression in the presence of vitamin D3 . CYP27B1 expression was not affected by vitamin D3 and inflammatory conditions.
Conclusions: This study indicates that the transcriptional activity of VDR is diminished under inflammatory conditions, which might mitigate the effectiveness of vitamin D3 supplementation during periodontal treatment.
Keywords: VDR; inflammation; mesenchymal stem cells; periodontal ligament; vitamin D.
© 2020 The Authors. Journal of Periodontology published by Wiley Periodicals LLC on behalf of American Academy of Periodontology.
Figures

Similar articles
-
Effect of vitamin D3 on the osteogenic differentiation of human periodontal ligament stromal cells under inflammatory conditions.J Periodontal Res. 2021 Jun;56(3):579-588. doi: 10.1111/jre.12858. Epub 2021 Feb 5. J Periodontal Res. 2021. PMID: 33547643 Free PMC article.
-
Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells.PLoS One. 2014 Feb 28;9(2):e90301. doi: 10.1371/journal.pone.0090301. eCollection 2014. PLoS One. 2014. PMID: 24587317 Free PMC article.
-
Extending the vitamin D pathway to vitamin D3 and CYP27A1 in periodontal ligament cells.J Periodontol. 2021 Jul;92(7):44-53. doi: 10.1002/JPER.20-0225. Epub 2020 Nov 10. J Periodontol. 2021. PMID: 33107041
-
Metabolism and Action of 25-Hydroxy-19-nor-Vitamin D₃ in Human Prostate Cells.Vitam Horm. 2016;100:357-77. doi: 10.1016/bs.vh.2015.10.009. Epub 2015 Dec 8. Vitam Horm. 2016. PMID: 26827959 Review.
-
Vitamin D status and gene transcription in immune cells.J Steroid Biochem Mol Biol. 2013 Jul;136:83-5. doi: 10.1016/j.jsbmb.2013.02.005. Epub 2013 Feb 13. J Steroid Biochem Mol Biol. 2013. PMID: 23416105 Review.
Cited by
-
Effect of vitamin D3 on the osteogenic differentiation of human periodontal ligament stromal cells under inflammatory conditions.J Periodontal Res. 2021 Jun;56(3):579-588. doi: 10.1111/jre.12858. Epub 2021 Feb 5. J Periodontal Res. 2021. PMID: 33547643 Free PMC article.
-
Effects of vitamin A in promoting proliferation and osteogenic differentiation of human periodontal ligament cells.In Vitro Cell Dev Biol Anim. 2023 Feb;59(2):121-130. doi: 10.1007/s11626-023-00754-6. Epub 2023 Mar 22. In Vitro Cell Dev Biol Anim. 2023. PMID: 36947388
-
Autophagy as a potential mechanism underlying the biological effect of 1,25-Dihydroxyvitamin D3 on periodontitis: a narrative review.BMC Oral Health. 2023 Feb 13;23(1):90. doi: 10.1186/s12903-023-02802-9. BMC Oral Health. 2023. PMID: 36782172 Free PMC article. Review.
-
Toll-Like Receptors and Dental Mesenchymal Stromal Cells.Front Oral Health. 2021 Apr 16;2:648901. doi: 10.3389/froh.2021.648901. eCollection 2021. Front Oral Health. 2021. PMID: 35048000 Free PMC article. Review.
-
Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria.Int J Mol Sci. 2025 Jul 21;26(14):7001. doi: 10.3390/ijms26147001. Int J Mol Sci. 2025. PMID: 40725246 Free PMC article.
References
-
- Perez‐Lopez FR. Vitamin D: the secosteroid hormone and human reproduction. Gynecol Endocrinol. 2007;23(1):13‐24. - PubMed
-
- Lips P. Vitamin D physiology. Prog Biophys Mol Bio. 2006;92(1):4‐8. - PubMed
-
- Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119‐134. - PubMed
-
- Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I. Skin is an autonomous organ in synthesis, two‐step activation and degradation of vitamin D(3): cYP27 in epidermis completes the set of essential vitamin D(3)‐hydroxylases. Steroids. 2001;66(3‐5):399‐408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

