Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary
- PMID: 32476019
- PMCID: PMC7456353
- DOI: 10.1093/humupd/dmaa018
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary
Abstract
Background: Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are found widespread in drinking water, foods, food packaging materials and other consumer products. Several PFAS have been identified as endocrine-disrupting chemicals based on their ability to interfere with normal reproductive function and hormonal signalling. Experimental models and epidemiologic studies suggest that PFAS exposures target the ovary and represent major risks for women's health.
Objective and rationale: This review summarises human population and toxicological studies on the association between PFAS exposure and ovarian function.
Search methods: A comprehensive review was performed by searching PubMed. Search terms included an extensive list of PFAS and health terms ranging from general keywords (e.g. ovarian, reproductive, follicle, oocyte) to specific keywords (including menarche, menstrual cycle, menopause, primary ovarian insufficiency/premature ovarian failure, steroid hormones), based on the authors' knowledge of the topic and key terms.
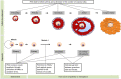
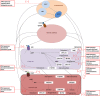
Outcomes: Clinical evidence demonstrates the presence of PFAS in follicular fluid and their ability to pass through the blood-follicle barrier. Although some studies found no evidence associating PFAS exposure with disruption in ovarian function, numerous epidemiologic studies, mostly with cross-sectional study designs, have identified associations of higher PFAS exposure with later menarche, irregular menstrual cycles, longer cycle length, earlier age of menopause and reduced levels of oestrogens and androgens. Adverse effects of PFAS on ovarian folliculogenesis and steroidogenesis have been confirmed in experimental models. Based on laboratory research findings, PFAS could diminish ovarian reserve and reduce endogenous hormone synthesis through activating peroxisome proliferator-activated receptors, disrupting gap junction intercellular communication between oocyte and granulosa cells, inducing thyroid hormone deficiency, antagonising ovarian enzyme activities involved in ovarian steroidogenesis or inhibiting kisspeptin signalling in the hypothalamus.
Wider implications: The published literature supports associations between PFAS exposure and adverse reproductive outcomes; however, the evidence remains insufficient to infer a causal relationship between PFAS exposure and ovarian disorders. Thus, more research is warranted. PFAS are of significant concern because these chemicals are ubiquitous and persistent in the environment and in humans. Moreover, susceptible groups, such as foetuses and pregnant women, may be exposed to harmful combinations of chemicals that include PFAS. However, the role environmental exposures play in reproductive disorders has received little attention by the medical community. To better understand the potential risk of PFAS on human ovarian function, additional experimental studies using PFAS doses equivalent to the exposure levels found in the general human population and mixtures of compounds are required. Prospective investigations in human populations are also warranted to ensure the temporality of PFAS exposure and health endpoints and to minimise the possibility of reverse causality.
Keywords: endocrine-disrupting chemicals (EDCs); folliculogenesis; ovary; perfluoroalkyl and polyfluoroalkyl substances (PFAS); steroidogenesis.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures

References
-
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for perfluoroalkyls. 2018. https://www.atsdr.cdc.gov/sites/peer_review/tox_profile_perfluoroalkyls.... - PubMed
-
- Anderson RH, Long GC, Porter RC, Anderson JK. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: field-validation of critical fate and transport properties. Chemosphere 2016;150:678–685. - PubMed
-
- Ateia M, Maroli A, Tharayil N, Karanfil T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: a review. Chemosphere 2019;220:866–882. - PubMed
-
- Atsma F, Bartelink M-LEL, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006;13:265–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

