Does the Sequence of Anthracycline and Taxane Matter? The NeoSAMBA Trial
- PMID: 32476183
- PMCID: PMC7485342
- DOI: 10.1634/theoncologist.2019-0805
Does the Sequence of Anthracycline and Taxane Matter? The NeoSAMBA Trial
Abstract
Background: Taxanes usually follow anthracyclines in breast cancer neo/adjuvant treatment, likely because of their later introduction into clinical practice. However, there is no biological rationale that justifies this current standard of care. We compared a taxane followed by an anthracycline-based regimen with the reverse sequence in the neoadjuvant setting.
Patients and methods: In a randomized, open-label, single-center phase II trial, women with inoperable, locally advanced, HER2-negative breast cancer were stratified by hormone receptor status and randomized to three cycles of docetaxel (T) followed by three cycles of fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus three cycles of FAC followed by three cycles of docetaxel. Surgery, radiotherapy, and adjuvant hormonal therapy were administered as per local guidelines. The primary endpoint was pathological complete response (pCR), and secondary endpoints included toxicity, event-free survival (EFS), and overall survival (OS).
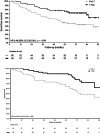
Results: Treatment sequence did not improve pCR, which was 7% with T-FAC and 3% with FAC-T. However, after a median follow-up of 79 months, the 5-year EFS rate was 75.7% (95% confidence interval [CI], 65.4%-87.7%) with T-FAC and 48.2% (95% CI, 37.0%-62.7%) with FAC-T (hazard ratio [HR], 0.46; 95% CI, 0.26-0.81; log-rank p = .0054), and the 5-year OS rate was 89.7% (95% CI, 82.2%-97.8%) with T-FAC and 64.7% (95% CI, 53.6%-78.1%) with FAC-T (HR, 0.41; 95% CI, 0.22-0.78; p = .0052). There were no unexpected toxicities.
Conclusion: We showed for the first time an improvement in EFS and OS with taxane-first compared with anthracycline-first sequencing chemotherapy in HER2-negative, locally advanced breast cancer. Confirmation of these results may have implications for clinical practice. This trial was registered with Clinicatrials.gov identifier NCT01270373.
Implications for practice: The NeoSAMBA trial showed a benefit for taxane-first sequencing chemotherapy consistent with the systematic review of the literature as well as the larger Neo-tAnGo study. Many recent and current ongoing clinical trials have already followed this treatment strategy. As a taxane-before-anthracycline sequence carries neither an incremental cost nor an increased toxicity, and given the available literature on this issue, reinforced that taxane-first regimen can be easily incorporated into daily clinical practice while awaiting confirmation of these findings from larger trials.
Keywords: Breast neoplasms; Doxorubicin; Taxoids.
© AlphaMed Press 2020.
Conflict of interest statement
Figures
References
-
- Bonadonna G, Brusamolino E, Valagussa P et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976;294:405–410. - PubMed
-
- Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005;353:1673–1684. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group . Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient‐level meta‐analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet Oncol 2019;393:1440–1452. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous