A comparative study of PF-06438179/GP1111 (an infliximab biosimilar) and reference infliximab in patients with moderate to severe active rheumatoid arthritis: A subgroup analysis
- PMID: 32476277
- PMCID: PMC7496806
- DOI: 10.1111/1756-185X.13846
A comparative study of PF-06438179/GP1111 (an infliximab biosimilar) and reference infliximab in patients with moderate to severe active rheumatoid arthritis: A subgroup analysis
Abstract
Aim: PF-06438179/GP1111 (PF-SZ-IFX) is a biosimilar of reference infliximab (Remicade® ). This analysis compared the efficacy of PF-SZ-IFX and reference infliximab sourced from the European Union (IFX-EU) in patient subgroups from a randomized, comparative study of PF-SZ-IFX versus IFX-EU.
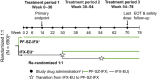
Methods: Patients with rheumatoid arthritis were randomized 1:1 to PF-SZ-IFX (n = 324) or IFX-EU (n = 326); study drug (3 mg/kg) was administered intravenously at weeks 0, 2, and 6, then every 8 weeks thereafter. Subgroup analyses of efficacy endpoints such as American College of Rheumatology criteria for ≥20% clinical improvement (ACR20), change in high-sensitivity C-reactive protein (hs-CRP), and change in Disease Activity Score in 28 joints, four components based on hs-CRP (DAS28-CRP) at weeks 14 and 30 were performed by age, gender, race, region, immunogenicity status, and treatment history.
Results: Overall, ACR20 response rates as well as changes in DAS28-CRP and hs-CRP at week 14 were similar between PF-SZ-IFX and IFX-EU within the subgroups of age, gender, race, region, treatment history, and immunogenicity status. Results to week 30 support overall similarity in efficacy between the two treatment arms in all subgroups.
Conclusion: Overall, PF-SZ-IFX and IFX-EU were similar in efficacy within the analyzed subgroups of age, gender, race, region, treatment history, and immunogenicity status. The efficacy results from these subgroup analyses were aligned with the previously described results for the overall population up to week 30.
Keywords: Japan; Latin America; arthritis - rheumatoid; biosimilar pharmaceuticals; infliximab.
© 2020 The Authors. International Journal of Rheumatic Diseases published by Asia Pacific League of Associations for Rheumatology.
Conflict of interest statement
HK reports grants from Pfizer; grants and personal fees from AbbVie GK, Asahi Kasei Pharma, Astellas Pharma Inc, Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma, and Novartis; and personal fees from Bristol‐Myers Squibb, Eli Lilly Japan KK, and Janssen Pharmaceutical KK. EU has no competing interests to disclose. TA reports personal fees for consulting from Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK, GlaxoSmithKline KK, Pfizer, and UCB Japan Co. Ltd; grants and personal fees for speakers’ bureaus from AbbVie Inc, Astellas Pharma Inc, Bristol‐Myers Squibb, Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan KK, Mitsubishi Tanabe Pharma Co., Otsuka Pharmaceutical Co. Ltd., Pfizer, Takeda Pharmaceutical Co. Ltd, and UCB Japan Co. Ltd; and grants from Alexion Pharmaceuticals, Inc. CA‐M reports personal fees for speakers’ bureaus from Pfizer, Lilly, Abbvie, and Roche. KK is an employee of Pfizer. TM, DPL, MIR, and MZ are employees of and own stock or options in Pfizer. SCR has received research grants from Pfizer.
Figures

Similar articles
-
Randomised, double-blind, phase III study comparing the infliximab biosimilar, PF-06438179/GP1111, with reference infliximab: efficacy, safety and immunogenicity from week 30 to week 54.RMD Open. 2019 Mar 28;5(1):e000876. doi: 10.1136/rmdopen-2018-000876. eCollection 2019. RMD Open. 2019. PMID: 30997153 Free PMC article. Clinical Trial.
-
Long-term Efficacy, Safety, and Immunogenicity of the Infliximab (IFX) Biosimilar, PF-06438179/GP1111, in Patients with Rheumatoid Arthritis After Switching from Reference IFX or Continuing Biosimilar Therapy: Week 54-78 Data From a Randomized, Double-Blind, Phase III Trial.BioDrugs. 2020 Apr;34(2):197-207. doi: 10.1007/s40259-019-00403-z. BioDrugs. 2020. PMID: 31939063 Free PMC article. Clinical Trial.
-
A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy.Arthritis Res Ther. 2018 Jul 27;20(1):155. doi: 10.1186/s13075-018-1646-4. Arthritis Res Ther. 2018. PMID: 30053896 Free PMC article. Clinical Trial.
-
PF-06438179/GP1111: An Infliximab Biosimilar.BioDrugs. 2018 Dec;32(6):639-642. doi: 10.1007/s40259-018-0310-5. BioDrugs. 2018. PMID: 30284704 Free PMC article. Review.
-
Infliximab biosimilar GP1111: a review of 5 years' post-approval experience.Expert Opin Biol Ther. 2024 Jul;24(7):615-625. doi: 10.1080/14712598.2024.2377298. Epub 2024 Jul 27. Expert Opin Biol Ther. 2024. PMID: 38976286 Review.
Cited by
-
[Use of biosimilars in the treatment of rheumatoid arthritis : An overview].Z Rheumatol. 2022 Mar;81(2):110-117. doi: 10.1007/s00393-021-01129-6. Epub 2021 Nov 26. Z Rheumatol. 2022. PMID: 34825948 Free PMC article. Review. German.
-
Characteristic analysis of adverse reactions of five anti-TNFɑ agents: a descriptive analysis from WHO-VigiAccess.Front Pharmacol. 2023 Jul 24;14:1169327. doi: 10.3389/fphar.2023.1169327. eCollection 2023. Front Pharmacol. 2023. PMID: 37554981 Free PMC article.
-
Biologic Drugs for Rheumatoid Arthritis in the Context of Biosimilars, Genetics, Epigenetics and COVID-19 Treatment.Cells. 2021 Feb 4;10(2):323. doi: 10.3390/cells10020323. Cells. 2021. PMID: 33557301 Free PMC article. Review.
-
Management of Rheumatoid Arthritis: An Overview.Cells. 2021 Oct 23;10(11):2857. doi: 10.3390/cells10112857. Cells. 2021. PMID: 34831081 Free PMC article. Review.
-
Biosimilars for the Treatment of Moderate to Severe Chronic Plaque Psoriasis.Psoriasis (Auckl). 2025 Aug 18;15:401-410. doi: 10.2147/PTT.S510156. eCollection 2025. Psoriasis (Auckl). 2025. PMID: 40860251 Free PMC article. Review.
References
-
- European Medicines Agency . Guideline on Similar Biological Medicinal Products. London: European Medicines Agency; 2014.
-
- US Food and Drug Administration . Scientific Considerations In Demonstrating Biosimilarity To A Reference Product: Guidance For Industry. Silver Spring, MD: US Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2015. .
-
- IMS Institute for Healthcare Informatics . Delivering on the Potential of Biosimilar Medicines: The Role of Functioning Competitive Markets. Parsippany, NJ: IMS Institute for Healthcare Informatics, 2016.
-
- Pfizer Inc . IXIFI (infliximab‐qbtx) Prescribing Information. New York. NY: Pfizer Inc; 2017.
-
- European Medicines Agency . Zessly: European public assessment report, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

