Predicting dyslipidemia after liver transplantation: A significant role of recipient metabolic inflammation profile
- PMID: 32476799
- PMCID: PMC7243645
- DOI: 10.3748/wjg.v26.i19.2374
Predicting dyslipidemia after liver transplantation: A significant role of recipient metabolic inflammation profile
Abstract
Background: Post-transplant dyslipidemia (PTDL) is a common complication in liver recipients and can cause morbidity and threaten graft function. The crosstalk between metabolic inflammation and dyslipidemia has been recently revealed. However, the role of grafts' and recipients' metabolic status in the development of PTDL has not been evaluated.
Aim: To investigate the association of recipients' metabolic inflammation status with PTDL and construct a predictive model.
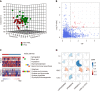
Methods: A total of 396 adult patients who received primary liver transplantation between 2015 and 2017 were enrolled. Metabolomics and cytokines were analyzed using recipients' pre-transplant peripheral blood in a training set (n = 72). An integrated prediction model was established according to the clinical risk factors and metabolic inflammation compounds and further verified in a validation set (n = 144).
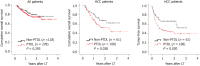
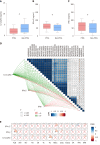
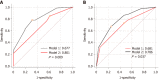
Results: The serum lipid profile took 3 mo to reach homeostasis after liver transplantation. A total of 278 (70.2%) liver recipients developed PTDL during a follow-up period of 1.78 (1.00, 2.97) years. The PTDL group showed a significantly lower tumor-free survival and overall survival than the non-PTDL group in patients with hepatocellular carcinoma (n = 169). The metabolomic analysis showed that metabolic features discriminating between the PTDL and non-PTDL groups were associated with lipid and glucose metabolism-associated pathways. Among metabolites and cytokines differentially expressed between the two groups, interleukin-12 (p70) showed the best diagnostic accuracy and significantly increased the predictive value when it was incorporated into the clinical model in both training and validation sets.
Conclusion: Recipients' pre-transplant serum interleukin-12 (p70) level is associated with the risk of PTDL and has potential clinical value for predicting PTDL.
Keywords: Cytokines; Dyslipidemia; Liver transplantation; Metabolomics; Predictive model.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that there is no conflict of interest to be disclosed.
Figures

References
-
- Chu KKW, Chan SC, Sin SL, Chan ACY, Chok KSH, Cheng IKP, Lo CM. Lipid profiles of donors and recipients of liver transplant: like father like son. Hepatol Int. 2017;11:300–305. - PubMed
-
- Parekh J, Corley DA, Feng S. Diabetes, hypertension and hyperlipidemia: prevalence over time and impact on long-term survival after liver transplantation. Am J Transplant. 2012;12:2181–2187. - PubMed
-
- Raees-Jalali G, Eshraghian A, Faghihi A, Roozbeh J, Sagheb MM, Eshraghian H, Behzadi S. Hyperlipidemia after kidney transplantation: long-term graft outcome. Iran J Kidney Dis. 2012;6:49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

