A common microbial signature is present in the lower airways of interstitial lung diseases including sarcoidosis
- PMID: 32476923
- PMCID: PMC7170129
- DOI: 10.36141/svdld.v35i4.7061
A common microbial signature is present in the lower airways of interstitial lung diseases including sarcoidosis
Abstract
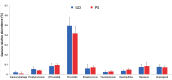
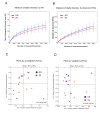
Background: The etiology of pulmonary sarcoidosis is not well established. Although the mechanism triggering pulmonary sarcoidosis remains to be established, inflammatory reactions seem to play an important role in this process. Objectives: The aim of this study was to define the composition of the lower airway microbiota in the bronchoalveolar lavage (BAL) of patients affected by interstitial lung diseases, including sarcoidosis, to determine whether the bacterial signature differs among these diseases. Methods: Ten patients affected by pulmonary sarcoidosis and 9 patients affected by other interstitial lung diseases were enrolled. 16S rRNA next-generation sequencing was used to study BAL microbial composition of these patients, and were also compared with already published microbial content in higher airways of such diseases. Results: Four phyla dominated the lower airway microbiota, Bacteroidetes being the most abundant phylum in both groups (56.9%). Diversity analysis showed no significant differences between the various diseases, particularly between pulmonary sarcoidosis and other interstitial lung diseases affecting lower airways. Conclusions: Our data indicate that the bacterial lower airways microbiota share the same signature and, therefore, cannot be used as a diagnostic tool to discriminate among different interstitial lung diseases, including sarcoidosis, while microbial diversity is present when considering lower or higher respiratory airways. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 354-362).
Keywords: airway microbiota; bronchoalveolar lavage (BAL); interstitial lung diseases; next generation sequencing; pulmonary sarcoidosis.
Copyright: © 2018 SARCOIDOSIS VASCULITIS AND DIFFUSE LUNG DISEASES.
Figures


References
-
- Chen ES, Moller DR. Sarcoidosis--scientific progress and clinical challenges. Nat Rev Rheumatol. 2011 Jul 12;7(8):457–67. - PubMed
-
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007 Nov 22;357(21):2153–65. - PubMed
-
- Medica I, Kastrin A, Maver A, Peterlin B. Role of genetic polymorphisms in ACE and TNF-alpha gene in sarcoidosis: a meta-analysis. J Hum Genet. 2007;52(10):836–847. - PubMed
-
- Müller-Quernheim J, Schürmann M, Hofmann S, Gaede KI, Fischer A, Prasse A, et al. Genetics of sarcoidosis. Clin Chest Med. 2008 Sep;29(3):391–414. - PubMed
-
- Sverrild A, Backer V, Kyvik KO, Kaprio J, Milman N, Svendsen CB, et al. Heredity in sarcoidosis: a registry-based twin study. Thorax. 2008 Oct;63(10):894–6. - PubMed
LinkOut - more resources
Full Text Sources
