Interstitial lung diseases associated with metal content in silicone breast implants: a case series
- PMID: 32476927
- PMCID: PMC7170128
- DOI: 10.36141/svdld.v35i4.7029
Interstitial lung diseases associated with metal content in silicone breast implants: a case series
Abstract
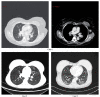
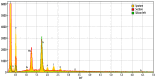
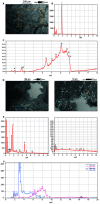
Background: Silicone gel-filled breast implants have been widely used for breast augmentation and reconstruction since the 1960's when the FDA approved them in women over 22 years of age. Concerns have been raised about the safety of those implants, with the focus upon whether silicone leak can spread to regional lymph nodes and remote organs and possibly cause inflammatory and immune responses. Objective: To present laboratory workup findings in 3 cases of interstitial lung diseases (ILD) linked with silicone implant leakage. Methods: ILD was diagnosed by tissue biopsy and Computerized Tomography (CT). We analyzed the metal content in both biological samples and raw material of the implants by means of scanning electron microscopy (SEM) and X-ray fluorescence (XRF). The metals lymphocyte proliferation analysis (MELISA®) was used to assess sensitization of the immune system to 19 metals. Results: The biological samples contained metals (silicon, nickel, zinc, tungsten, iron, aluminum, and zirconium) which are also present in the silicone implant. The MELISA test showed sensitization to nickel, zinc and tin. Limitations: Some of the immunogenic metals present in the implant were under the limits of detection of SEM and XRF and the sensitivity of MELISA test is unknown. Conclusions: The laboratory assessments of the 3 herein described women indicated that their interstitial lung disease was associated with the metal content of their silicone gel-filled breast implantations. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 381-389).
Keywords: breast implant; metals; pulmonary disease; silicone.
Copyright: © 2018 SARCOIDOSIS VASCULITIS AND DIFFUSE LUNG DISEASES.
Figures
References
-
- Cronin TD, Gerow FJ. Augmentation mammoplasty: a new ‘natural feel’ prosthesis. Transections of the Third International Congress of Plastic Surgery, Amsterdam. Excerpt Medical. 1964:41–9.
-
- Cunningham B. The Mentor Core study on silicone Memory Gel breast implants. Plast Reconstr Surg. 2007;120(Suppl 1):19S–29S. - PubMed
-
- Holmich LR, Kjoller K, Vejborg I, et al. Prevalence of silicone breast implant rupture among Danish women. Plast Reconstr Surg. 2001;108:848–58. - PubMed
-
- Jaume Alijotas-Reig J, Esteve-Valverde E, Gil-Aliberas N, Gimenez VC. Autoimmune/inflammatory syndrome induced by adjuvants – ASIA – related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunologic Research. 2018;66(1120-14) - PubMed
-
- Singh N, Picha GJ, Hardas B, Schumacher A, Murphy DK. Five-year safety data for more than 55,000 subjects following breast implantation: comparison of rare adverse event rates with silicone implants versus national norms and saline implants. Plast Reconstr Surg. 2017;140(4):666–79. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
