Emerging Roles of Activity-Dependent Alternative Splicing in Homeostatic Plasticity
- PMID: 32477067
- PMCID: PMC7235277
- DOI: 10.3389/fncel.2020.00104
Emerging Roles of Activity-Dependent Alternative Splicing in Homeostatic Plasticity
Abstract
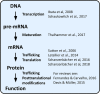
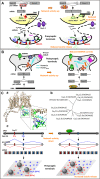
Homeostatic plasticity refers to the ability of neuronal networks to stabilize their activity in the face of external perturbations. Most forms of homeostatic plasticity ultimately depend on changes in the expression or activity of ion channels and synaptic proteins, which may occur at the gene, transcript, or protein level. The most extensively investigated homeostatic mechanisms entail adaptations in protein function or localization following activity-dependent posttranslational modifications. Numerous studies have also highlighted how homeostatic plasticity can be achieved by adjusting local protein translation at synapses or transcription of specific genes in the nucleus. In comparison, little attention has been devoted to whether and how alternative splicing (AS) of pre-mRNAs underlies some forms of homeostatic plasticity. AS not only expands proteome diversity but also contributes to the spatiotemporal dynamics of mRNA transcripts. Prominent in the brain where it can be regulated by neuronal activity, it is a flexible process, tightly controlled by a multitude of factors. Given its extensive use and versatility in optimizing the function of ion channels and synaptic proteins, we argue that AS is ideally suited to achieve homeostatic control of neuronal output. We support this thesis by reviewing emerging evidence linking AS to various forms of homeostatic plasticity: homeostatic intrinsic plasticity, synaptic scaling, and presynaptic homeostatic plasticity. Further, we highlight the relevance of this connection for brain pathologies.
Keywords: P/Q-type Ca2+ channels; alternative splicing; homeostatic plasticity; homer1; repressor element 1 silencing transcription factor (REST).
Copyright © 2020 Thalhammer, Jaudon and Cingolani.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

