Dopamine Modulates Excitatory Synaptic Transmission by Activating Presynaptic D1-like Dopamine Receptors in the RA Projection Neurons of Zebra Finches
- PMID: 32477072
- PMCID: PMC7235289
- DOI: 10.3389/fncel.2020.00126
Dopamine Modulates Excitatory Synaptic Transmission by Activating Presynaptic D1-like Dopamine Receptors in the RA Projection Neurons of Zebra Finches
Abstract
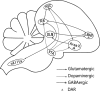
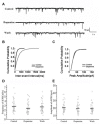
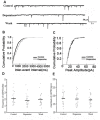
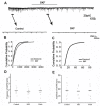
Songbirds are useful vertebrate study models for vocal learning and memory. The robust nucleus of the arcopallium (RA) receives synaptic inputs from both the posterior and anterior pathways of the song control system in songbirds. Hence, RA plays an important role in the control of singing. RA receives dopaminergic (DArgic) inputs that increase the excitability of RA projection neurons (PNs). However, the effects of DA on excitatory synaptic transmission are yet to be deciphered. In this study, the effects of DA on the excitatory synaptic transmission of the PNs in the RA of adult male zebra finches were investigated using a whole-cell patch-clamp recording. We observed that DA decreased the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) and miniature excitatory postsynaptic currents (mEPSCs). The effects of DA were mimicked by the D1-like DA receptor (D1R) agonist, SKF-38393, but not the D2-like DA receptor (D2R) agonist, Quinpirole. Also, the effects of DA were blocked by D1R antagonist, SCH-23390, but not the D2R antagonist, Sulpiride. These results demonstrate that DA modulates excitatory synaptic transmission by acting on D1R in the RA of adult male zebra finches.
Keywords: D1-like dopamine receptors; dopamine; excitatory synaptic transmission; the robust nucleus of the arcopallium; zebra finch.
Copyright © 2020 Wang, Liu, Wang, Sun, Yao, Li and Meng.
Figures


Similar articles
-
Dopamine modulates the excitability of projection neurons in the robust nucleus of the arcopallium in adult zebra finches.PLoS One. 2013 Dec 5;8(12):e82497. doi: 10.1371/journal.pone.0082497. eCollection 2013. PLoS One. 2013. PMID: 24340033 Free PMC article.
-
BDNF enhances electrophysiological activity and excitatory synaptic transmission of RA projection neurons in adult male zebra finches.Brain Res. 2023 Feb 15;1801:148208. doi: 10.1016/j.brainres.2022.148208. Epub 2022 Dec 19. Brain Res. 2023. PMID: 36549361
-
Sex differences of excitatory synaptic transmission in RA projection neurons of adult zebra finches.Neurosci Lett. 2014 Oct 17;582:75-80. doi: 10.1016/j.neulet.2014.09.001. Epub 2014 Sep 8. Neurosci Lett. 2014. PMID: 25220700
-
Activation of D1-like dopamine receptors increases the NMDA-induced gain modulation through a PKA-dependent pathway in the premotor nucleus of adult zebra finches.Neurosci Lett. 2015 Mar 4;589:37-41. doi: 10.1016/j.neulet.2015.01.032. Epub 2015 Jan 14. Neurosci Lett. 2015. PMID: 25596438
-
Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons.J Neurosci. 2006 Sep 27;26(39):10043-50. doi: 10.1523/JNEUROSCI.1819-06.2006. J Neurosci. 2006. Retraction in: J Neurosci. 2012 Jun 27;32(26):9116. doi: 10.1523/JNEUROSCI.1889-12.2012. PMID: 17005867 Free PMC article. Retracted.
Cited by
-
Estradiol decreases the excitability of RA projection neurons in adult male zebra finches.Front Cell Neurosci. 2023 Feb 14;17:1046984. doi: 10.3389/fncel.2023.1046984. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36866064 Free PMC article.
-
Analogies of human speech and bird song: From vocal learning behavior to its neural basis.Front Psychol. 2023 Feb 22;14:1100969. doi: 10.3389/fpsyg.2023.1100969. eCollection 2023. Front Psychol. 2023. PMID: 36910811 Free PMC article. Review.
-
Antidepressant-like effects of cinnamamide derivative M2 via D2 receptors in the mouse medial prefrontal cortex.Acta Pharmacol Sin. 2022 Sep;43(9):2267-2275. doi: 10.1038/s41401-021-00854-7. Epub 2022 Jan 25. Acta Pharmacol Sin. 2022. PMID: 35079131 Free PMC article.
-
Neural mechanism of dopamine modulating singing related behavior in songbirds: an updated review.PeerJ. 2025 Jun 5;13:e19500. doi: 10.7717/peerj.19500. eCollection 2025. PeerJ. 2025. PMID: 40487051 Free PMC article. Review.
-
Cell type specializations of the vocal-motor cortex in songbirds.Cell Rep. 2023 Nov 28;42(11):113344. doi: 10.1016/j.celrep.2023.113344. Epub 2023 Oct 30. Cell Rep. 2023. PMID: 37910500 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

