Gender Differences in Neurodegeneration, Neuroinflammation and Na+-Ca2+ Exchangers in the Female A53T Transgenic Mouse Model of Parkinson's Disease
- PMID: 32477098
- PMCID: PMC7232579
- DOI: 10.3389/fnagi.2020.00118
Gender Differences in Neurodegeneration, Neuroinflammation and Na+-Ca2+ Exchangers in the Female A53T Transgenic Mouse Model of Parkinson's Disease
Abstract
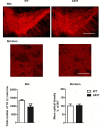
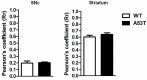
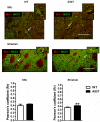
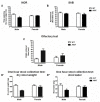
Twelve-month-old male mice expressing the human A53T variant of α-synuclein (A53T) develop dopamine neuron degeneration, neuroinflammation, and motor deficits, along with dysfunctions of the mitochondrial Na+-Ca2+ exchanger (NCX) isoforms 1 (NCX1) and 3 (NCX3) in the nigrostriatal system. Since gender is thought to play a role in the etiology of Parkinson's disease (PD), we characterized neurochemical and behavioral alterations in 12-month-old female A53T transgenic mice. We investigated the presence of dopaminergic degeneration, astrogliosis and microgliosis using immunohistochemistry for tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule-1 (IBA-1) in both the substantia nigra pars compacta (SNc) and striatum. In the same regions, we also evaluated the co-localization of NCX1 in cells positive for IBA-1 and the co-localization of NCX3 in TH-positive neurons and fibers. Furthermore, in both male and female mice, we performed motor (beam walking and pole tests) and memory [novel object recognition (NOR) and spontaneous alternation] tasks, together with tests to evaluate peripheral deficits (olfactory and stool collection tests). Female A53T transgenic mice displayed degeneration of nigral dopaminergic neurons, but neither microgliosis nor astrogliosis in the SNc and striatum. Moreover, female A53T transgenic mice displayed co-localization between NCX1 and IBA-1 positive cells in the striatum but not SNc, whereas NCX3 did not co-localize with either TH-positive terminals or neuronal bodies in the nigrostriatal system. Furthermore, female A53T transgenic mice showed increased crossing time in the beam walking test, but no impairments in the pole or memory tests, and in tests that evaluated peripheral deficits, whereas male A53T transgenic mice displayed motor, memory and peripheral deficits. Immunohistochemical and behavioral results obtained here in the female mice differ from those previously observed in males, and suggest a dissimilar influence of NCX1 and NCX3 on dopaminergic function in female and male A53T transgenic mice, strengthening the validity of these mice as a model for studying the etiological factors of PD.
Keywords: GFAP; IBA-1; NCXs; constipation; dopamine; memory; midbrain; striatum.
Copyright © 2020 Costa, Sisalli, Simola, Della Notte, Casu, Serra, Pinna, Feliciello, Annunziato, Scorziello and Morelli.
Figures


Similar articles
-
NCX1 and NCX3 as potential factors contributing to neurodegeneration and neuroinflammation in the A53T transgenic mouse model of Parkinson's Disease.Cell Death Dis. 2018 Jun 25;9(7):725. doi: 10.1038/s41419-018-0775-7. Cell Death Dis. 2018. PMID: 29941946 Free PMC article.
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Ncx3-Induced Mitochondrial Dysfunction in Midbrain Leads to Neuroinflammation in Striatum of A53t-α-Synuclein Transgenic Old Mice.Int J Mol Sci. 2021 Jul 30;22(15):8177. doi: 10.3390/ijms22158177. Int J Mol Sci. 2021. PMID: 34360942 Free PMC article.
-
A53T-alpha-synuclein-overexpression in the mouse nigrostriatal pathway leads to early increase of 14-3-3 epsilon and late increase of GFAP.J Neural Transm (Vienna). 2012 Mar;119(3):297-312. doi: 10.1007/s00702-011-0717-3. Epub 2011 Sep 30. J Neural Transm (Vienna). 2012. PMID: 21960009 Free PMC article.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
Cited by
-
Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson's disease.Front Neurosci. 2023 May 5;17:1146979. doi: 10.3389/fnins.2023.1146979. eCollection 2023. Front Neurosci. 2023. PMID: 37214398 Free PMC article.
-
Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson's Disease.Biomedicines. 2024 Jan 8;12(1):130. doi: 10.3390/biomedicines12010130. Biomedicines. 2024. PMID: 38255235 Free PMC article.
-
Hippocampal network hyperexcitability in young transgenic mice expressing human mutant alpha-synuclein.Neurobiol Dis. 2021 Feb;149:105226. doi: 10.1016/j.nbd.2020.105226. Epub 2020 Dec 30. Neurobiol Dis. 2021. PMID: 33347975 Free PMC article.
-
An antioxidant and anti-ER stress combination therapy elevates phosphorylation of α-Syn at serine 129 and alleviates post-TBI PD-like pathology in a sex-specific manner in mice.Exp Neurol. 2024 Jul;377:114795. doi: 10.1016/j.expneurol.2024.114795. Epub 2024 Apr 23. Exp Neurol. 2024. PMID: 38657855 Free PMC article.
-
Genotype-Based Housing as a Potential Confounder in Studies Using Transgenic Mouse Models-Insight from the A53T Mouse Model of Parkinson's Disease.Biomedicines. 2025 Jun 19;13(6):1506. doi: 10.3390/biomedicines13061506. Biomedicines. 2025. PMID: 40564225 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

