Comparison of Donepezil, Memantine, Melatonin, and Liuwei Dihuang Decoction on Behavioral and Immune Endocrine Responses of Aged Senescence-Accelerated Mouse Resistant 1 Mice
- PMID: 32477103
- PMCID: PMC7241684
- DOI: 10.3389/fphar.2020.00350
Comparison of Donepezil, Memantine, Melatonin, and Liuwei Dihuang Decoction on Behavioral and Immune Endocrine Responses of Aged Senescence-Accelerated Mouse Resistant 1 Mice
Abstract
Aging is a natural biological process associated with cognitive decline and neuroendocrine-immune system changes; the neuroendocrine-immune system plays crucial role in brain aging and neurodegeneration, and it is essential to discern beneficial attempts to delay the aging progress based on immunological aging. In this study, we have investigated the effects of Traditional Chinese Medicine (TCM)-Liuwei Dihuang decoction (LW)-and donepezil, memantine, and melatonin on cognitive decline in aging mice. The aged SAMR1 mice received oral administration of donepezil (1mg/kg), memantine (10 mg/kg), melatonin (10 mg/kg), and LW (10 g/kg) for 3 months. A shuttle box, Morris water maze, and elevated-zero maze were performed to assess cognitive function, and flowcytometry, Luminex, and radioimmunoassay were performed to measure the lymphocyte subsets, inflammatory factors, and hormones. We observed that survival days of mice was increased with melatonin and LW, the anxiety behavior was significantly improved by memantine, melatonin, and LW treatment, active avoidance responses significantly improved by LW, donepezil, and memantine, the spatial learning ability was significantly improved by donepezil, and LW and melatonin were beneficial to the spatial memory of old mice. For immune function, LW increased CD4+ and CD4+CD28+ cells and reduced TNF-α, IL-1β, and G-CSF in plasma, and it also promoted the secretion of anti-inflammatory factors IL-4, IL-5, and IL-10 by regulating the active of Th2 cells in spleen. Donepezil and memantine exerted protective effects against CD4+CD28+ cell decrease caused by aging and reduced the pro-inflammatory factors TNF-α, IL-1β, and G-CSF in plasma. Melatonin could reverse CD8+CD28+ cell imbalances and increased B cells. For endocrine factors, LW increased TSH levels in the pituitary, and melatonin increased the GH level in blood. Our findings indicated that LW improved the cognitive decline in aging mice, and this might be associated with modulation of the active T cells and HPG axis hormones as well as increasing anti-inflammatory factors. Meanwhile, donepezil and memantine have advantages in regulating adaptive immunity, melatonin has advantages in the regulation of B cells and pituitary hormones, and LW exhibits a better effect on neuroendocrine immune function compared with the others from a holistic point of view. LW might be a potential therapeutic strategy for anti-aging-related syndromes, and it can also provide a value on medication guidance about drug combinations or treatment in clinic.
Keywords: Liuwei Dihuang decoction; aging; cognition; immune response; inflammation.
Copyright © 2020 Zeng, Zhang, Wang, Cheng, Zhang and Zhou.
Figures

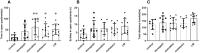



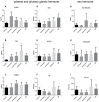

Similar articles
-
LW-AFC, A New Formula Derived from Liuwei Dihuang Decoction, Ameliorates Cognitive Deterioration and Modulates Neuroendocrine-Immune System in SAMP8 Mouse.Curr Alzheimer Res. 2017;14(2):221-238. doi: 10.2174/1567205013666160603001637. Curr Alzheimer Res. 2017. PMID: 27335033
-
LW-AFC, a new formula derived from Liuwei Dihuang decoction, ameliorates behavioral and pathological deterioration via modulating the neuroendocrine-immune system in PrP-hAβPPswe/PS1ΔE9 transgenic mice.Alzheimers Res Ther. 2016 Dec 13;8(1):57. doi: 10.1186/s13195-016-0226-6. Alzheimers Res Ther. 2016. PMID: 27964740 Free PMC article.
-
The anti-aging effects of LW-AFC via correcting immune dysfunctions in senescence accelerated mouse resistant 1 (SAMR1) strain.Oncotarget. 2016 May 10;7(19):26949-65. doi: 10.18632/oncotarget.8877. Oncotarget. 2016. PMID: 27105505 Free PMC article.
-
LW-AFC, a new formula from the traditional Chinese medicine Liuwei Dihuang decoction, as a promising therapy for Alzheimer's disease: Pharmacological effects and mechanisms.Adv Pharmacol. 2020;87:159-177. doi: 10.1016/bs.apha.2019.10.005. Epub 2020 Jan 3. Adv Pharmacol. 2020. PMID: 32089232 Review.
-
Effect of Liuwei Dihuang decoction, a traditional Chinese medicinal prescription, on the neuroendocrine immunomodulation network.Pharmacol Ther. 2016 Jun;162:170-8. doi: 10.1016/j.pharmthera.2016.02.004. Epub 2016 Feb 16. Pharmacol Ther. 2016. PMID: 26896567 Review.
Cited by
-
Donepezil Ameliorates Pulmonary Arterial Hypertension by Inhibiting M2-Macrophage Activation.Front Cardiovasc Med. 2021 Mar 15;8:639541. doi: 10.3389/fcvm.2021.639541. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33791350 Free PMC article.
-
Identification of the Active Compound of Liu Wei Di Huang Wan for Treatment of Gestational Diabetes Mellitus via Network Pharmacology and Molecular Docking.J Diabetes Res. 2022 May 28;2022:4808303. doi: 10.1155/2022/4808303. eCollection 2022. J Diabetes Res. 2022. PMID: 35669396 Free PMC article.
-
Unlocking the potential of exercise: harnessing myokines to delay musculoskeletal aging and improve cognitive health.Front Physiol. 2024 Sep 2;15:1338875. doi: 10.3389/fphys.2024.1338875. eCollection 2024. Front Physiol. 2024. PMID: 39286235 Free PMC article. Review.
-
Application and Effectiveness of Chinese Medicine in Regulating Immune Checkpoint Pathways.Chin J Integr Med. 2023 Nov;29(11):1045-1056. doi: 10.1007/s11655-023-3743-8. Epub 2023 Aug 15. Chin J Integr Med. 2023. PMID: 37580466 Review.
-
Network Pharmacology and Experimental Validation to Reveal the Pharmacological Mechanisms of Liuwei Dihuang Decoction Against Intervertebral Disc Degeneration.Drug Des Devel Ther. 2021 Dec 2;15:4911-4924. doi: 10.2147/DDDT.S338439. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34880601 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

