A Physiology-Based Pharmacokinetic Framework to Support Drug Development and Dose Precision During Therapeutic Hypothermia in Neonates
- PMID: 32477113
- PMCID: PMC7237643
- DOI: 10.3389/fphar.2020.00587
A Physiology-Based Pharmacokinetic Framework to Support Drug Development and Dose Precision During Therapeutic Hypothermia in Neonates
Abstract
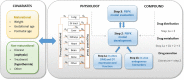
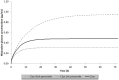
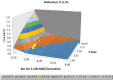
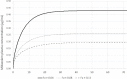
Therapeutic hypothermia (TH) is standard treatment for neonates (≥36 weeks) with perinatal asphyxia (PA) and hypoxic-ischemic encephalopathy. TH reduces mortality and neurodevelopmental disability due to reduced metabolic rate and decreased neuronal apoptosis. Since both hypothermia and PA influence physiology, they are expected to alter pharmacokinetics (PK). Tools for personalized dosing in this setting are lacking. A neonatal hypothermia physiology-based PK (PBPK) framework would enable precision dosing in the clinic. In this literature review, the stepwise approach, benefits and challenges to develop such a PBPK framework are covered. It hereby contributes to explore the impact of non-maturational PK covariates. First, the current evidence as well as knowledge gaps on the impact of PA and TH on drug absorption, distribution, metabolism and excretion in neonates is summarized. While reduced renal drug elimination is well-documented in neonates with PA undergoing hypothermia, knowledge of the impact on drug metabolism is limited. Second, a multidisciplinary approach to develop a neonatal hypothermia PBPK framework is presented. Insights on the effect of hypothermia on hepatic drug elimination can partly be generated from in vitro (human/animal) profiling of hepatic drug metabolizing enzymes and transporters. Also, endogenous biomarkers may be evaluated as surrogate for metabolic activity. To distinguish the impact of PA versus hypothermia on drug metabolism, in vivo neonatal animal data are needed. The conventional pig is a well-established model for PA and the neonatal Göttingen minipig should be further explored for PA under hypothermia conditions, as it is the most commonly used pig strain in nonclinical drug development. Finally, a strategy is proposed for establishing and fine-tuning compound-specific PBPK models for this application. Besides improvement of clinical exposure predictions of drugs used during hypothermia, the developed PBPK models can be applied in drug development. Add-on pharmacotherapies to further improve outcome in neonates undergoing hypothermia are under investigation, all in need for dosing guidance. Furthermore, the hypothermia PBPK framework can be used to develop temperature-driven PBPK models for other populations or indications. The applicability of the proposed workflow and the challenges in the development of the PBPK framework are illustrated for midazolam as model drug.
Keywords: drug metabolism; neonate; pharmacokinetics; physiology-based pharmacokinetic modelling; therapeutic hypothermia.
Copyright © 2020 Smits, Annaert, Van Cruchten and Allegaert.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

