Patient Perspectives on the Therapeutic Profile of Botulinum Neurotoxin Type A in Spasticity
- PMID: 32477251
- PMCID: PMC7233119
- DOI: 10.3389/fneur.2020.00388
Patient Perspectives on the Therapeutic Profile of Botulinum Neurotoxin Type A in Spasticity
Erratum in
-
Corrigendum: Patient Perspectives on the Therapeutic Profile of Botulinum Neurotoxin Type A in Spasticity.Front Neurol. 2020 Dec 17;11:629181. doi: 10.3389/fneur.2020.629181. eCollection 2020. Front Neurol. 2020. PMID: 33391178 Free PMC article.
Abstract
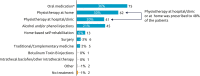
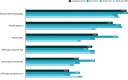
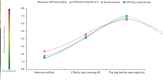
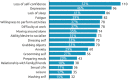
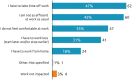
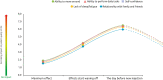
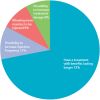
Background: Botulinum toxin-A (BoNT-A) injections are first-line treatment for adult spasticity. Prior patient surveys have reported that BoNT-A treatment improves quality of life but that symptoms usually recur before the next injection. We aimed to explore, in-depth, patient perceptions of the impact of spasticity and the waning of BoNT-A therapeutic effects. Methods: An internet-based survey was conducted through Carenity, an online patient community, from May to September 2019 in France, Germany, Italy, UK and USA. Eligible respondents were adult patients with spasticity due to stroke, traumatic brain injury (TBI) or spinal cord injury (SCI) who had ≥2 previous BoNT-A injections. Results: Two hundred and ten respondents (mean 47.2 years) met screening criteria and had their responses analyzed. Overall, 43% of respondents had spasticity due to stroke, 30% due to TBI and 27% due to SCI. The mean [95% CI] injection frequency for spasticity management was 3.6 [3.4-3.7] injections/year. Respondents described the time profile of their response to BoNT-A. The mean reported onset of therapeutic effect was 12.9 [12.1-13.7] days and the mean time to peak effect was 5.0 [4.7-5.4] weeks. Symptom re-emergence between injections was common (83%); the time from injection to symptom re-emergence was 89.4 [86.3-92.4] days. Muscle spasms usually re-emerge first (64%), followed by muscle stiffness or rigidity (40%), and limb pain (20%). Over half (52%) of respondents said they had lost their self-confidence, 46% experienced depression and 41% experienced a lack of sleep due to their spasticity symptoms in the past 12 months. Following a report of symptom re-emergence, the most common management approaches were to add adjunctive treatments (36%), increase the BoNT-A dose (28%), and wait for the next injection (26%). Seventy two percentage of respondents said they would like a longer lasting BoNT-A treatment. Conclusions: Patients with spasticity can expect a characteristic profile of BoNT-A effects, namely time lag to onset and peak effect followed by a gradual decline in the symptomatic benefits. Symptom re-emergence is common and has significant impact on quality of life. Greater patient/clinician awareness of this therapeutic profile should lead to better level of overall satisfaction with treatment, informed therapeutic discussions and treatment schedule planning.
Keywords: botulinum toxin; patient perspectives; patient survey; spasticity; waning of effect.
Copyright © 2020 Jacinto, Varriale, Pain, Lysandropoulos and Esquenazi.
Figures

References
-
- RCP Spasticity in Adults: Management Using Botulinum Toxin. National guidelines (2018). Available online at https://www.rcplondon.ac.uk/guidelines-policy/spasticity-adults-manageme... (accessed April 17, 2020).
LinkOut - more resources
Full Text Sources
Miscellaneous

