Causes and Consequences of Hypertriglyceridemia
- PMID: 32477261
- PMCID: PMC7239992
- DOI: 10.3389/fendo.2020.00252
Causes and Consequences of Hypertriglyceridemia
Abstract
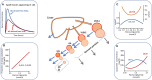
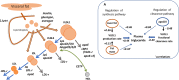
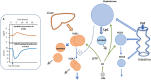
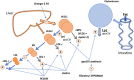
Elevations in plasma triglyceride are the result of overproduction and impaired clearance of triglyceride-rich lipoproteins-very low-density lipoproteins (VLDL) and chylomicrons. Hypertriglyceridemia is characterized by an accumulation in the circulation of large VLDL-VLDL1-and its lipolytic products, and throughout the VLDL-LDL delipidation cascade perturbations occur that give rise to increased concentrations of remnant lipoproteins and small, dense low-density lipoprotein (LDL). The elevated risk of atherosclerotic cardiovascular disease in hypertriglyceridemia is believed to result from the exposure of the artery wall to these aberrant lipoprotein species. Key regulators of the metabolism of triglyceride-rich lipoproteins have been identified and a number of these are targets for pharmacological intervention. However, a clear picture is yet to emerge as to how to relate triglyceride lowering to reduced risk of atherosclerosis.
Keywords: VLDL; apoB; chylomicron; lipid; metabolism.
Copyright © 2020 Packard, Boren and Taskinen.
Figures

References
-
- Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. (2011) 32:1345–61. 10.1016/S1567-5688(11)70033-2 - DOI - PMC - PubMed
-
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2017) 38:2459–72. 10.1093/eurheartj/ehx144 - DOI - PMC - PubMed
-
- Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon J, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. Pathophysiological, genetic and therapeutic insights. a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2020). ehz962. 10.1093/eurheartj/ehz962 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

