Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders
- PMID: 32477322
- PMCID: PMC7232541
- DOI: 10.3389/fimmu.2020.00648
Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders
Abstract
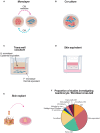
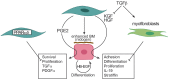
Background/Objective: Skin fibrosis is the result of aberrant processes leading to abnormal deposition of extracellular matrix (ECM) in the dermis. In healthy skin, keratinocytes participate to maintain skin homeostasis by actively crosstalking with fibroblasts. Within the wide spectrum of fibrotic skin disorders, relatively little attention has been devoted to the role of keratinocytes for their capacity to participate to skin fibrosis. This systematic review aims at summarizing the available knowledge on the reciprocal interplay of keratinocytes with fibroblasts and their soluble mediators in physiological states, mostly wound healing, and conditions associated with skin fibrosis. Methods: We performed a systematic literature search on PubMed to identify in vitro and ex vivo human studies investigating the keratinocyte characteristics and their interplay with fibroblasts in physiological conditions and within fibrotic skin disorders including hypertrophic scars, keloids, and systemic sclerosis. Studies were selected according to pre-specified eligibility criteria. Data on study methods, models, stimuli and outcomes were retrieved and summarized according to pre-specified criteria. Results: Among the 6,271 abstracts retrieved, 73 articles were included, of which 14 were specifically dealing with fibrotic skin pathologies. Fifty-six studies investigated how keratinocyte may affect fibroblast responses in terms of ECM-related genes or protein production, phenotype modification, and cytokine production. Most studies in both physiological conditions and fibrosis demonstrated that keratinocytes stimulate fibroblasts through the production of interleukin 1, inducing keratinocyte growth factor (KGF) and metalloproteinases in the fibroblasts. When the potential of keratinocytes to modulate collagen synthesis by healthy fibroblasts was explored, the results were controversial. Nevertheless, studies investigating keratinocytes from fibrotic skin, including keloids, hypertrophic scar, and scleroderma, suggested their potential involvement in enhancing ECM deposition. Twenty-three papers investigated keratinocyte proliferation differentiation and production of soluble mediators in response to interactions with fibroblasts. Most studies showed that fibroblasts modulate keratinocyte viability, proliferation, and differentiation. The production of KGF by fibroblast was identified as key for these functions. Conclusions: This review condenses evidence for the active interaction between keratinocytes and fibroblasts in maintaining skin homeostasis and the altered homeostatic interplay between keratinocytes and dermal fibroblasts in scleroderma and scleroderma-like disorders.
Keywords: cytokine; extracellular matrix; fibroblast; fibrosis; homeostasis; keratinocyte; systemic sclerosis.
Copyright © 2020 Russo, Brembilla and Chizzolini.
Figures

References
-
- Boin F, Chizzolini C. Inflammation and immunity. In: Varga J, Denton C, Wigley F, Allanore Y, Kuwana M, editors. Scleroderma: From Pathogenesis to Comprehensive Management. New York, NY: Springer; (2017). p. 161–96.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

