Concurrent Immune Suppression and Hyperinflammation in Patients With Community-Acquired Pneumonia
- PMID: 32477337
- PMCID: PMC7232566
- DOI: 10.3389/fimmu.2020.00796
Concurrent Immune Suppression and Hyperinflammation in Patients With Community-Acquired Pneumonia
Erratum in
-
Corrigendum: Concurrent Immune Suppression and Hyperinflammation in Patients With Community-Acquired Pneumonia.Front Immunol. 2020 Nov 27;11:626667. doi: 10.3389/fimmu.2020.626667. eCollection 2020. Front Immunol. 2020. PMID: 33329617 Free PMC article.
Abstract
Background: The nature and timing of the host immune response during infections remain uncertain and most knowledge is derived from critically ill sepsis patients. We aimed to test the hypothesis that community-acquired pneumonia (CAP) is associated with concurrent immune suppression and systemic inflammation.
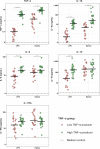
Methods: Blood was collected from 79 CAP patients within 24 h after hospitalization and 1 month after discharge; 42 age- and sex-matched subjects without acute infection served as controls. Blood leukocytes were stimulated with lipopolysaccharide (LPS) or Klebsiella pneumoniae, and cytokines were measured in supernatants. Fifteen plasma biomarkers reflective of key host response pathways were compared between CAP patients with the strongest immune suppression (lowest 25% blood leukocyte tumor necrosis factor (TNF)-α production in response to LPS) and those with the least immune suppression (highest 25% of LPS-induced TNF-α production).
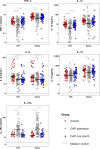
Results: Blood leukocytes of CAP patients (relative to control subjects) showed a reduced capacity to release TNF-α, interleukin (IL)-1β, IL-6 and IL-10 upon stimulation with LPS or K. pneumoniae, with a concurrently enhanced ability to release the anti-inflammatory mediator IL-1 receptor antagonist, irrespective of the presence of sepsis (18.9% of cases). Low (relative to high) TNF-α producers displayed higher plasma levels of biomarkers reflecting systemic inflammation, neutrophil degranulation, endothelial cell activation, a disturbed vascular barrier function and coagulation activation.
Conclusion: CAP replicates a common feature of immune suppression in sepsis. The coexistence of immune suppression and hyperinflammation in CAP argues against the theory of two distinct phases during the host response to sepsis.
Keywords: community-acquired pneumonia; immune suppression; lipopolysaccharide; sepsis; systemic inflammation.
Copyright © 2020 Brands, Haak, Klarenbeek, Otto, Faber, Lutter, Scicluna, Wiersinga and van der Poll.
Figures

References
-
- World Health Organization [WHO] The Top 10 Causes of Death. (2018). Available online at: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed May 24, 2018)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

