Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches
- PMID: 32477347
- PMCID: PMC7235343
- DOI: 10.3389/fimmu.2020.00849
Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches
Abstract
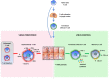
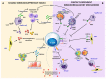
A great effort of research has been devoted in the last few years to developing new anti-HBV therapies of finite duration that also provide effective sustained control of virus replication and antigen production. Among the potential therapeutic strategies, immune-modulation represents a promising option to cure HBV infection and the adaptive immune response is a rational target for novel therapeutic interventions, in consideration of the key role played by T cells in the control of virus infections. HBV-specific T cells are severely dysfunctional in chronic HBV infection as a result of several inhibitory mechanisms which are simultaneously active within the chronically inflamed liver. Indeed, the liver is a tolerogenic organ harboring different non-parenchymal cell populations which can serve as antigen presenting cells (APC) but are poorly efficient in effector T cell priming, with propensity to induce T cell tolerance rather than T cell activation, because of a poor expression of co-stimulatory molecules, up-regulation of the co-inhibitory ligands PD-L1 and PD-L2 upon IFN stimulation, and production of immune regulatory cytokines, such as IL10 and TGF-β. They include resident dendritic cells (DCs), comprising myeloid and plasmacytoid DCs, liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), hepatic stellate cells (HSCs) as well as the hepatocytes themselves. Additional regulatory mechanisms which contribute to T cell attrition in the chronically infected liver are the high levels of soluble mediators, such as arginase, indoleamine 2,3-dioxygenase (IDO) and suppressive cytokines, the up-regulation of inhibitory checkpoint receptor/ligand pairs, the expansion of regulatory cells, such as CD4+FOXp3+ Treg cells, myeloid-derived suppressor cells and NK cells. This review will deal with the interactions between immune cells and liver environment discussing the different mechanisms which contribute to T cell dysfunction in chronic hepatitis B, some of which are specifically activated in HBV infection and others which are instead common to chronic inflammatory liver diseases in general. Therapeutic interventions targeting dysregulated pathways and cellular functions will be also delineated.
Keywords: T cell exhaustion; chronic HBV infection; immune-therapy; immunoregulatory mechanisms; liver environment.
Copyright © 2020 Fisicaro, Barili, Rossi, Montali, Vecchi, Acerbi, Laccabue, Zecca, Penna, Missale, Ferrari and Boni.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

