Characterization of the Porcine CLEC12A and Analysis of Its Expression on Blood Dendritic Cell Subsets
- PMID: 32477350
- PMCID: PMC7237735
- DOI: 10.3389/fimmu.2020.00863
Characterization of the Porcine CLEC12A and Analysis of Its Expression on Blood Dendritic Cell Subsets
Abstract
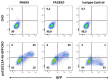
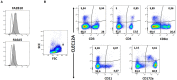
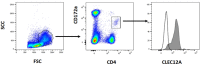
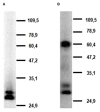
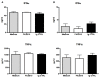
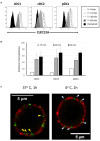
CLEC12A has been proposed as a suitable target for delivering antigen to dendritic cells (DCs) to enhance vaccine efficacy both in human and mouse. In this study, we have characterized the porcine homolog of CLEC12A (poCLEC12A). Using new monoclonal antibodies (mAb), raised against its ectodomain, poCLEC12A was found to be expressed on alveolar macrophages, blood conventional type 1 and type 2 DCs and plasmacytoid DCs, but not on monocytes, T cells, B cells or NK cells, in contrast to its human and murine homologs. Western blot analysis showed that in alveolar macrophages this receptor is expressed both as a monomer and a dimer. After binding to DCs, anti- poCLEC12A mAb was efficiently internalized. No significant changes were observed in TNFα or IFNα secretion by plasmacytoid DCs stimulated with either CpGs (ODN2216) or polyinosinic-polycytidylic acid (poly I:C), upon incubation with mAb. These results provide the basis for future investigations aimed to assess the ability of anti-poCLEC12A mAbs to improve vaccine efficacy by targeting antigen to DCs.
Keywords: C-type lectin; CLEC12A; dendritic cell; monoclonal antibody; pig.
Copyright © 2020 Álvarez, Nieto-Pelegrín, Martínez de la Riva, Toki, Poderoso, Revilla, Uenishi, Ezquerra and Domínguez.
Figures

Similar articles
-
Porcine CLEC12B is expressed on alveolar macrophages and blood dendritic cells.Dev Comp Immunol. 2020 Oct;111:103767. doi: 10.1016/j.dci.2020.103767. Epub 2020 Jun 11. Dev Comp Immunol. 2020. PMID: 32535044
-
The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses.J Immunol. 2009 Jun 15;182(12):7587-94. doi: 10.4049/jimmunol.0900464. J Immunol. 2009. PMID: 19494282
-
Antibody-mediated targeting of antigen to C-type lectin-like receptors Clec9A and Clec12A elicits different vaccination outcomes.Mol Immunol. 2017 Jan;81:143-150. doi: 10.1016/j.molimm.2016.12.010. Epub 2016 Dec 12. Mol Immunol. 2017. PMID: 27978488
-
CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses.J Immunol. 2016 Oct 1;197(7):2715-25. doi: 10.4049/jimmunol.1600011. Epub 2016 Aug 26. J Immunol. 2016. PMID: 27566820
-
In vivo cancer vaccination: Which dendritic cells to target and how?Cancer Treat Rev. 2018 Dec;71:88-101. doi: 10.1016/j.ctrv.2018.10.012. Epub 2018 Oct 25. Cancer Treat Rev. 2018. PMID: 30390423 Free PMC article. Review.
Cited by
-
Tolerogenic Immunotherapy: Targeting DC Surface Receptors to Induce Antigen-Specific Tolerance.Front Immunol. 2021 Feb 19;12:643240. doi: 10.3389/fimmu.2021.643240. eCollection 2021. Front Immunol. 2021. PMID: 33679806 Free PMC article. Review.
-
Expanding the Known Repertoire of C-Type Lectin Receptors Binding to Toxoplasma gondii Oocysts Using a Modified High-Resolution Immunofluorescence Assay.mSphere. 2021 Mar 31;6(2):e01341-20. doi: 10.1128/mSphere.01341-20. mSphere. 2021. PMID: 33789945 Free PMC article.
-
CLEC4A and CLEC12B C-type lectin receptors mediate interactions with Pneumocystis cell wall components.J Med Microbiol. 2023 Jun;72(6):001714. doi: 10.1099/jmm.0.001714. J Med Microbiol. 2023. PMID: 37294293 Free PMC article.
-
Antigen Targeting of Porcine Skin DEC205+ Dendritic Cells.Vaccines (Basel). 2022 Apr 26;10(5):684. doi: 10.3390/vaccines10050684. Vaccines (Basel). 2022. PMID: 35632440 Free PMC article.
-
Intratumoral injection of caerin 1.1 and 1.9 peptides increases the efficacy of vaccinated TC-1 tumor-bearing mice with PD-1 blockade by modulating macrophage heterogeneity and the activation of CD8+ T cells in the tumor microenvironment.Clin Transl Immunology. 2021 Aug 17;10(8):e1335. doi: 10.1002/cti2.1335. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34429969 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

