Long-term outcomes of monascin - a novel dual peroxisome proliferator-activated receptor γ/nuclear factor-erythroid 2 related factor-2 agonist in experimental intracerebral hemorrhage
- PMID: 32477427
- PMCID: PMC7232052
- DOI: 10.1177/1756286420921083
Long-term outcomes of monascin - a novel dual peroxisome proliferator-activated receptor γ/nuclear factor-erythroid 2 related factor-2 agonist in experimental intracerebral hemorrhage
Abstract
Background: Hematoma is the chief culprit in brain injury following intracranial cerebral hemorrhage (ICH). Noninvasive hematoma clearance could be an option to prevent and alleviate early brain injury after ICH. Peroxisome proliferator-activated receptor γ (PPAR-γ) and nuclear factor-erythroid 2 related factor-2 (Nrf2) facilitate removal of hematoma in ICH. Monascin acts as the natural Nrf2 activator with PPAR-γ agonist, and the long-term effects of monascin following ICH have not been elucidated.
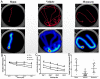
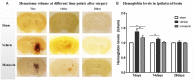
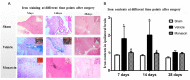
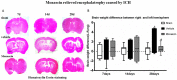
Methods: ICH in rats was induced by stereotactic, intrastriatal injection of type IV collagenase. Monascin was administered twice daily by gastric perfusion for 14 days after ICH induction. Long-term neurological scores (T maze, Garcia scales, rotor rod test, and Morris water maze), hematoma volume, as well as iron overload around hematoma and brain atrophy were evaluated at 7, 14, and 28 days after ICH.
Results: The results showed that monascin improved long-term neurological deficits, spatial memory performance, learning ability, and brain shrinkage after ICH. Monascin also reduced hematoma volume at 7 days and iron content at 7 and 14 days after ICH.
Conclusion: PPAR γ and Nrf2 play a crucial role in hematoma clearance after ICH in rat. As a dual agonist of PPAR γ and Nrf2, monascin improved long-term outcomes by facilitating hematoma clearance, and by attenuating iron overload and brain atrophy after experimental ICH.
Keywords: dual receptor agonist; hematoma clearance; intracerebral hemorrhage; monascin; peroxisome proliferator-activated receptor γ/nuclear factor-erythroid 2 related factor-2.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Regulation of nuclear factor erythroid-2-related factor 2 as a potential therapeutic target in intracerebral hemorrhage.Front Mol Neurosci. 2022 Sep 29;15:995518. doi: 10.3389/fnmol.2022.995518. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36245922 Free PMC article. Review.
-
The effect of monascin on hematoma clearance and edema after intracerebral hemorrhage in rats.Brain Res Bull. 2017 Sep;134:24-29. doi: 10.1016/j.brainresbull.2017.06.018. Epub 2017 Jun 24. Brain Res Bull. 2017. PMID: 28655601
-
Neuroprotection by Nrf2 via modulating microglial phenotype and phagocytosis after intracerebral hemorrhage.Heliyon. 2023 Feb 16;9(2):e13777. doi: 10.1016/j.heliyon.2023.e13777. eCollection 2023 Feb. Heliyon. 2023. PMID: 36852060 Free PMC article.
-
PPAR-γ Promotes Hematoma Clearance through Haptoglobin-Hemoglobin-CD163 in a Rat Model of Intracerebral Hemorrhage.Behav Neurol. 2018 Jul 9;2018:7646104. doi: 10.1155/2018/7646104. eCollection 2018. Behav Neurol. 2018. PMID: 30123388 Free PMC article.
-
Pleiotropic role of PPARγ in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-κB.CNS Neurosci Ther. 2015 Apr;21(4):357-66. doi: 10.1111/cns.12350. Epub 2014 Nov 28. CNS Neurosci Ther. 2015. PMID: 25430543 Free PMC article. Review.
Cited by
-
Mechanisms of memory impairment in animal models of nontraumatic intracranial hemorrhage: A systematic review of the literature.Brain Hemorrhages. 2022 Jun;3(2):77-93. doi: 10.1016/j.hest.2021.08.002. Epub 2021 Aug 10. Brain Hemorrhages. 2022. PMID: 36093312 Free PMC article.
-
Research progress of endogenous hematoma absorption after intracerebral hemorrhage.Front Neurol. 2023 Mar 10;14:1115726. doi: 10.3389/fneur.2023.1115726. eCollection 2023. Front Neurol. 2023. PMID: 36970539 Free PMC article. Review.
-
Elucidating the progress and impact of ferroptosis in hemorrhagic stroke.Front Cell Neurosci. 2023 Jan 11;16:1067570. doi: 10.3389/fncel.2022.1067570. eCollection 2022. Front Cell Neurosci. 2023. PMID: 36713782 Free PMC article. Review.
-
Insight into Crosstalk between Ferroptosis and Necroptosis: Novel Therapeutics in Ischemic Stroke.Oxid Med Cell Longev. 2021 Jun 25;2021:9991001. doi: 10.1155/2021/9991001. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34257829 Free PMC article. Review.
-
Regulation of nuclear factor erythroid-2-related factor 2 as a potential therapeutic target in intracerebral hemorrhage.Front Mol Neurosci. 2022 Sep 29;15:995518. doi: 10.3389/fnmol.2022.995518. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36245922 Free PMC article. Review.
References
-
- Babu R, Bagley JH, Di C, et al. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg Focus 2012; 32: E8. - PubMed
-
- Wang G, Hu W, Tang Q, et al. Effect comparison of both iron chelators on outcomes, iron deposit, and iron transporters after intracerebral hemorrhage in rats. Mol Neurobiol 2016; 53: 3576–3585. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

