Optimal Ablation Techniques for Ventricular Tachycardia Management
- PMID: 32477780
- PMCID: PMC7252666
- DOI: 10.19102/icrm.2018.090101
Optimal Ablation Techniques for Ventricular Tachycardia Management
Abstract
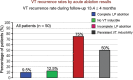
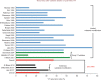
Ventricular arrhythmias arise from complex electroanatomical substrates in patients with structural heart disease. There have been significant advancements in technologies and techniques for ventricular tachycardia catheter ablation. A systematic approach to mapping and ablation is paramount, and catheter ablation has shifted to be a potential first-line therapy for patients needing early intervention, particularly for those with post-infarction arrhythmias. Furthermore, imaging integration, coupled with a systematic, detailed substrate characterization, has shown promise and provides a safe and effective approach for long-term arrhythmia control.
Keywords: Ablation; image integration; mapping; substrate characterization.
Copyright: © 2018 Innovations in Cardiac Rhythm Management.
Conflict of interest statement
The authors report no conflicts of interest for the published content.
Figures


References
-
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. [CrossRef] [PubMed] - DOI - PMC - PubMed
-
- The Antiarrhythmics versus Implantable Defibrillator (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias (AVID) N Engl J Med. 1997;337(22):1576–1583. [CrossRef] [PubMed] - DOI - PubMed
-
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. [CrossRef] [PubMed] - DOI - PubMed
-
- Sweeney MO, Sherfesee L, DeGroot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverterdefibrillator patients. Heart Rhythm. 2010;7(3):353–360. [CrossRef] [PubMed] - DOI - PubMed
-
- Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–1017. [CrossRef] [PubMed] - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
