Differential Expression of Putative Ornithodoros turicata Defensins Mediated by Tick Feeding
- PMID: 32477960
- PMCID: PMC7232577
- DOI: 10.3389/fcimb.2020.00152
Differential Expression of Putative Ornithodoros turicata Defensins Mediated by Tick Feeding
Erratum in
-
Corrigendum: Differential Expression of Putative Ornithodoros turicata Defensins Mediated by Tick Feeding.Front Cell Infect Microbiol. 2020 Jul 2;10:310. doi: 10.3389/fcimb.2020.00310. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32714879 Free PMC article.
Abstract
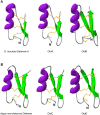
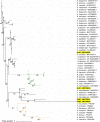
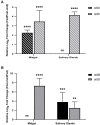
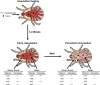
Additional research on soft ticks in the family Argasidae is needed to bridge the knowledge gap relative to hard ticks of the family Ixodidae; especially, the molecular mechanisms of Ornithodoros biology. Ornithodoros species are vectors of human and animal pathogens that include tick-borne relapsing fever spirochetes and African swine fever virus. Soft tick vector-pathogen interactions involving components of the tick immune response are not understood. Ticks utilize a basic innate immune system consisting of recognition factors and cellular and humoral responses to produce antimicrobial peptides, like defensins. In the present study, we identified and characterized the first putative defensins of Ornithodoros turicata, an argasid tick found primarily in the southwestern United States and regions of Latin America. Four genes (otdA, otdB, otdC, and otdD) were identified through sequencing and their predicted amino acid sequences contained motifs characteristic of arthropod defensins. A phylogenetic analysis grouped these four genes with arthropod defensins, and computational structural analyses further supported the identification. Since pathogens transmitted by O. turicata colonize both the midgut and salivary glands, expression patterns of the putative defensins were determined in these tissues 1 week post engorgement and after molting. Defensin genes up-regulated in the tick midgut 1 week post blood feeding were otdA and otdC, while otdD was up-regulated in the midgut of post-molt ticks. Moreover, otdB and otdD were also up-regulated in the salivary glands of flat post-molt ticks, while otdC was up-regulated within 1 week post blood-feeding. This work is foundational toward additional studies to determine mechanisms of vector competence and pathogen transmission from O. turicata.
Keywords: Ornithdoros turicata; antimicrobial peptide (AMP); argasid (soft) ticks; defensins; gene expression; immune response.
Copyright © 2020 Armstrong, Kneubehl, Mitchell, Krishnavajhala, Teel, Pérez de León and Lopez.
Figures

Similar articles
-
Vector Competence of Geographical Populations of Ornithodoros turicata for the Tick-Borne Relapsing Fever Spirochete Borrelia turicatae.Appl Environ Microbiol. 2018 Oct 17;84(21):e01505-18. doi: 10.1128/AEM.01505-18. Print 2018 Nov 1. Appl Environ Microbiol. 2018. PMID: 30143510 Free PMC article.
-
Imaging of Borrelia turicatae Producing the Green Fluorescent Protein Reveals Persistent Colonization of the Ornithodoros turicata Midgut and Salivary Glands from Nymphal Acquisition through Transmission.Appl Environ Microbiol. 2017 Feb 15;83(5):e02503-16. doi: 10.1128/AEM.02503-16. Print 2017 Mar 1. Appl Environ Microbiol. 2017. PMID: 27986725 Free PMC article.
-
Identification and characterization of a histamine-binding lipocalin-like molecule from the relapsing fever tick Ornithodoros turicata.Insect Mol Biol. 2018 Apr;27(2):177-187. doi: 10.1111/imb.12362. Epub 2017 Nov 22. Insect Mol Biol. 2018. PMID: 29164729
-
Reviewing the Potential Vectors and Hosts of African Swine Fever Virus Transmission in the United States.Vector Borne Zoonotic Dis. 2019 Jul;19(7):512-524. doi: 10.1089/vbz.2018.2387. Epub 2019 Feb 19. Vector Borne Zoonotic Dis. 2019. PMID: 30785371 Free PMC article. Review.
-
Historical overview and update on relapsing fever group Borrelia in Latin America.Parasit Vectors. 2022 Jun 8;15(1):196. doi: 10.1186/s13071-022-05289-5. Parasit Vectors. 2022. PMID: 35676728 Free PMC article. Review.
Cited by
-
Defensins as a promising class of tick antimicrobial peptides: a scoping review.Infect Dis Poverty. 2022 Jun 20;11(1):71. doi: 10.1186/s40249-022-00996-8. Infect Dis Poverty. 2022. PMID: 35725522 Free PMC article.
-
Detection of African Swine Fever Virus in Ornithodoros Tick Species Associated with Indigenous and Extralimital Warthog Populations in South Africa.Viruses. 2022 Jul 26;14(8):1617. doi: 10.3390/v14081617. Viruses. 2022. PMID: 35893686 Free PMC article.
-
Identification of the Gene Repertoire of the IMD Pathway and Expression of Antimicrobial Peptide Genes in Several Tissues and Hemolymph of the Cockroach Blattella germanica.Int J Mol Sci. 2022 Jul 30;23(15):8444. doi: 10.3390/ijms23158444. Int J Mol Sci. 2022. PMID: 35955579 Free PMC article.
References
-
- Balashov Y. S. (1972). Bloodsucking ticks (Ixodoidea)- vectors of diseases of man and animals. Misc. Publ. Entomol. Soc. Am. 8, 161–376.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

