African Swine Fever Virus: An Emerging DNA Arbovirus
- PMID: 32478103
- PMCID: PMC7237725
- DOI: 10.3389/fvets.2020.00215
African Swine Fever Virus: An Emerging DNA Arbovirus
Abstract
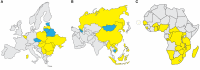
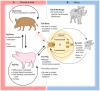
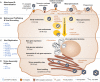
African swine fever virus (ASFV) is the sole member of the family Asfarviridae, and the only known DNA arbovirus. Since its identification in Kenya in 1921, ASFV has remained endemic in Africa, maintained in a sylvatic cycle between Ornithodoros soft ticks and warthogs (Phacochoerus africanus) which do not develop clinical disease with ASFV infection. However, ASFV causes a devastating and economically significant disease of domestic (Sus scrofa domesticus) and feral (Sus scrofa ferus) swine. There is no ASFV vaccine available, and current control measures consist of strict animal quarantine and culling procedures. The virus is highly stable and easily spreads by infected swine, contaminated pork products and fomites, or via transmission by the Ornithodoros vector. Competent Ornithodoros argasid soft tick vectors are known to exist not only in Africa, but also in parts of Europe and the Americas. Once ASFV is established in the argasid soft tick vector, eradication can be difficult due to the long lifespan of Ornithodoros ticks and their proclivity to inhabit the burrows of warthogs or pens and shelters of domestic pigs. Establishment of endemic ASFV infections in wild boar populations further complicates the control of ASF. Between the late 1950s and early 1980s, ASFV emerged in Europe, Russia and South America, but was mostly eradicated by the mid-1990s. In 2007, a highly virulent genotype II ASFV strain emerged in the Caucasus region and subsequently spread into the Russian Federation and Europe, where it has continued to circulate and spread. Most recently, ASFV emerged in China and has now spread to several neighboring countries in Southeast Asia. The high morbidity and mortality associated with ASFV, the lack of an efficacious vaccine, and the complex makeup of the ASFV virion and genome as well as its lifecycle, make this pathogen a serious threat to the global swine industry and national economies. Topics covered by this review include factors important for ASFV infection, replication, maintenance, and transmission, with attention to the role of the argasid tick vector and the sylvatic transmission cycle, current and future control strategies for ASF, and knowledge gaps regarding the virus itself, its vector and host species.
Keywords: African swine fever virus; DNA arbovirus; domestic swine; soft tick; transmission; virus replication; wild boar.
Copyright © 2020 Gaudreault, Madden, Wilson, Trujillo and Richt.
Figures
References
-
- Montgomery RE. On a form of swine fever occurring in British East Africa. J Comp Pathol. (1921) 34:59–191. 10.1016/S0368-1742(21)80031-4 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

