Multilayer corrosion-resistant material based on iron-carbon alloys
- PMID: 32478192
- PMCID: PMC7248665
- DOI: 10.1016/j.heliyon.2020.e04039
Multilayer corrosion-resistant material based on iron-carbon alloys
Abstract
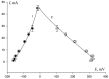
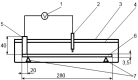
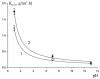
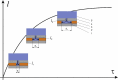
In this study, the architecture of a multilayer metallic material of iron-carbon alloys with an internal protector was developed based on theoretical studies. The operability of the proposed architecture was experimentally verified using gravimetry and electrochemical analysis. The internal position of the protector enabled the modification of the mechanism of corrosion. The stages of corrosion of the multilayer material were revealed; the material was observed as useable until the third layer was perforated. To demonstrate the obtained results, the authors conducted a set of experiments using X-ray microscopy and scanning electron microscopy with an electron probe analysis of the chemical composition. The cost of the developed material is within the same range as widely used corrosion-resistant stainless austenite steels; and in terms of corrosion resistance, this material is comparable to palladium, molybdenum, nickel, and Hastelloy.
Keywords: Corrosion; Electrochemical potential; Mass corrosion index; Materials science; Multilayer materials; Pitting; Protector.
© 2020 The Authors.
Figures



References
-
- Elanskii G.N., Semin A.E., Paderin S.N., Shakhov S.I., Shevelev L.N. Results of 14th steelmakers congress. Metallurgist. 2017;1:29–45.
-
- Nemenov A.M. Events in figures and facts. Metallurgist. 2017;7:95–104.
-
- Pavlov A.A. Development of new corrosion-resistant bimetals with increased corrosion resistance prepared by electroslag surfacing technology. Chem. Petrol. Eng. 2017;53(7-8):551–556.
-
- Agarwal D.C. Nickel base alloys and newer 6Mo stainless steels meet corrosion challenges of the modern day chemical process industrie. Anti-Corros. Methods Mater. 2001;48(5):287–297.
LinkOut - more resources
Full Text Sources

