Catalytic and Photochemical Strategies to Stabilized Radicals Based on Anomeric Nucleophiles
- PMID: 32479072
- PMCID: PMC9640225
- DOI: 10.1021/jacs.0c03298
Catalytic and Photochemical Strategies to Stabilized Radicals Based on Anomeric Nucleophiles
Abstract
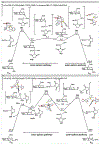
Carbohydrates, one of the three primary macromolecules of living organisms, play significant roles in various biological processes such as intercellular communication, cell recognition, and immune activity. While the majority of established methods for the installation of carbohydrates through the anomeric carbon rely on nucleophilic displacement, anomeric radicals represent an attractive alternative because of their functional group compatibility and high anomeric selectivities. Herein, we demonstrate that anomeric nucleophiles such as C1 stannanes can be converted into anomeric radicals by merging Cu(I) catalysis with blue light irradiation to achieve highly stereoselective C(sp3)-S cross-coupling reactions. Mechanistic studies and DFT calculations revealed that the C-S bond-forming step occurs via the transfer of the anomeric radical directly to a sulfur electrophile bound to Cu(II) species. This pathway complements a radical chain observed for photochemical metal-free conditions where a disulfide initiator can be activated by a Lewis base additive. Both strategies utilize anomeric nucleophiles as efficient radical donors and achieve a switch from an ionic to a radical pathway. Taken together, the stability of glycosyl nucleophiles, a broad substrate scope, and high anomeric selectivities observed for the thermal and photochemical protocols make this novel C-S cross coupling a practical tool for late-stage glycodiversification of bioactive natural products and drug candidates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Direct, stereoselective thioglycosylation enabled by an organophotoredox radical strategy.Chem Sci. 2020 Oct 19;11(48):13079-13084. doi: 10.1039/d0sc04136j. Chem Sci. 2020. PMID: 34094490 Free PMC article.
-
Glycosyl Cross-Coupling of Anomeric Nucleophiles: Scope, Mechanism, and Applications in the Synthesis of Aryl C-Glycosides.J Am Chem Soc. 2017 Dec 13;139(49):17908-17922. doi: 10.1021/jacs.7b08707. Epub 2017 Nov 30. J Am Chem Soc. 2017. PMID: 29148749 Free PMC article.
-
Rethinking Carbohydrate Synthesis: Stereoretentive Reactions of Anomeric Stannanes.Chemistry. 2019 Mar 1;25(13):3147-3155. doi: 10.1002/chem.201803082. Epub 2018 Dec 13. Chemistry. 2019. PMID: 30051523 Free PMC article.
-
2-nitroglycals as powerful glycosyl donors: application in the synthesis of biologically important molecules.Acc Chem Res. 2008 Aug;41(8):1059-73. doi: 10.1021/ar7002495. Epub 2008 Jul 4. Acc Chem Res. 2008. PMID: 18598060 Review.
-
A brief account of the application of glycosyl halide as glycosyl radical precursor towards glycosylation through visible light catalysis.Carbohydr Res. 2025 Aug;554:109537. doi: 10.1016/j.carres.2025.109537. Epub 2025 May 17. Carbohydr Res. 2025. PMID: 40409016 Review.
Cited by
-
Light-Mediated Cross-Coupling of Anomeric Trifluoroborates.Org Lett. 2021 Jun 4;23(11):4289-4293. doi: 10.1021/acs.orglett.1c01035. Epub 2021 May 24. Org Lett. 2021. PMID: 34029464 Free PMC article.
-
Transformations of carbohydrate derivatives enabled by photocatalysis and visible light photochemistry.Chem Sci. 2024 Jan 2;15(4):1204-1236. doi: 10.1039/d3sc05400d. eCollection 2024 Jan 24. Chem Sci. 2024. PMID: 38274059 Free PMC article. Review.
-
Stereoselective alkyl C-glycosylation of glycosyl esters via anomeric C-O bond homolysis: efficient access to C-glycosyl amino acids and C-glycosyl peptides.Chem Sci. 2023 Jun 20;14(27):7569-7580. doi: 10.1039/d3sc01995k. eCollection 2023 Jul 12. Chem Sci. 2023. PMID: 37449071 Free PMC article.
-
Direct, stereoselective thioglycosylation enabled by an organophotoredox radical strategy.Chem Sci. 2020 Oct 19;11(48):13079-13084. doi: 10.1039/d0sc04136j. Chem Sci. 2020. PMID: 34094490 Free PMC article.
-
Synthesis of C-acyl furanosides via the cross-coupling of glycosyl esters with carboxylic acids.Chem Sci. 2021 Jul 23;12(34):11414-11419. doi: 10.1039/d1sc03596g. eCollection 2021 Sep 1. Chem Sci. 2021. PMID: 34667550 Free PMC article.
References
-
- Varki A; Cummings RD; Esko JD; Freeze HH; Stanley P; Bertozzi CR; Hart GW; Etzler ME Essentials of Glycobiology; Cold Spring Harbor Laboratory Press, 2009. - PubMed
-
- Cecioni S; Imberty A; Vidal S Glycomimetics versus Multivalent Glycoconjugates for the Design of High Affinity Lectin Ligands. Chem. Rev 2015, 115, 525–561. - PubMed
-
- Hagen B; Vorm S; Hansen T; Marel GA; Codée JDC Stereoselective Glycosylations–Additions to Oxocarbenium Ions. In Selective Glycosylations: Synthetic Methods and Catalysts; Bennett CS, Ed.; Wiley, 2017; pp 1–28.
-
- Yang Y; Yu B Recent Advances in the Chemical Synthesis of C-Glycosides. Chem. Rev 2017, 117, 12281–12356. - PubMed
-
- Giese B; Dupuis J DiastereoseIective syntheses of C-glycopyranosides. Angew. Chem., Int. Ed. Engl 1983, 22, 622.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

