Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks
- PMID: 32479493
- PMCID: PMC7263567
- DOI: 10.1371/journal.pgen.1008717
Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks
Abstract
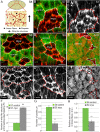
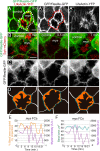
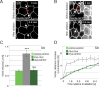
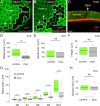
Forces generated by the actomyosin cytoskeleton are key contributors to many morphogenetic processes. The actomyosin cytoskeleton organises in different types of networks depending on intracellular signals and on cell-cell and cell-extracellular matrix (ECM) interactions. However, actomyosin networks are not static and transitions between them have been proposed to drive morphogenesis. Still, little is known about the mechanisms that regulate the dynamics of actomyosin networks during morphogenesis. This work uses the Drosophila follicular epithelium, real-time imaging, laser ablation and quantitative analysis to study the role of integrins on the regulation of basal actomyosin networks organisation and dynamics and the potential contribution of this role to cell shape. We find that elimination of integrins from follicle cells impairs F-actin recruitment to basal medial actomyosin stress fibers. The available F-actin redistributes to the so-called whip-like structures, present at tricellular junctions, and into a new type of actin-rich protrusions that emanate from the basal cortex and project towards the medial region. These F-actin protrusions are dynamic and changes in total protrusion area correlate with periodic cycles of basal myosin accumulation and constriction pulses of the cell membrane. Finally, we find that follicle cells lacking integrin function show increased membrane tension and reduced basal surface. Furthermore, the actin-rich protrusions are responsible for these phenotypes as their elimination in integrin mutant follicle cells rescues both tension and basal surface defects. We thus propose that the role of integrins as regulators of stress fibers plays a key role on controlling epithelial cell shape, as integrin disruption promotes reorganisation into other types of actomyosin networks, in a manner that interferes with proper expansion of epithelial basal surfaces.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Basal actomyosin pulses expand epithelium coordinating cell flattening and tissue elongation.Nat Commun. 2024 Apr 8;15(1):3000. doi: 10.1038/s41467-024-47236-1. Nat Commun. 2024. PMID: 38589403 Free PMC article.
-
Local weakening of cell-extracellular matrix adhesion triggers basal epithelial tissue folding.EMBO J. 2025 Apr;44(7):2002-2024. doi: 10.1038/s44318-025-00384-6. Epub 2025 Feb 17. EMBO J. 2025. PMID: 39962267 Free PMC article.
-
Apical Sarcomere-like Actomyosin Contracts Nonmuscle Drosophila Epithelial Cells.Dev Cell. 2016 Nov 7;39(3):346-358. doi: 10.1016/j.devcel.2016.09.023. Epub 2016 Oct 20. Dev Cell. 2016. PMID: 27773487 Free PMC article.
-
Epithelial polarity and morphogenesis.Curr Opin Cell Biol. 2011 Oct;23(5):540-6. doi: 10.1016/j.ceb.2011.07.005. Epub 2011 Jul 30. Curr Opin Cell Biol. 2011. PMID: 21807488 Review.
-
Stresses at the cell surface during animal cell morphogenesis.Curr Biol. 2014 May 19;24(10):R484-94. doi: 10.1016/j.cub.2014.03.059. Curr Biol. 2014. PMID: 24845681 Review.
Cited by
-
Actin polymerization and depolymerization in developing vertebrates.Front Physiol. 2023 Sep 8;14:1213668. doi: 10.3389/fphys.2023.1213668. eCollection 2023. Front Physiol. 2023. PMID: 37745245 Free PMC article. Review.
-
Integrins Can Act as Suppressors of Ras-Mediated Oncogenesis in the Drosophila Wing Disc Epithelium.Cancers (Basel). 2023 Nov 15;15(22):5432. doi: 10.3390/cancers15225432. Cancers (Basel). 2023. PMID: 38001693 Free PMC article.
-
Regionalized regulation of actomyosin organization influences cardiomyocyte cell shape changes during chamber curvature formation.bioRxiv [Preprint]. 2025 Apr 16:2025.01.07.631779. doi: 10.1101/2025.01.07.631779. bioRxiv. 2025. PMID: 39829878 Free PMC article. Preprint.
-
Tensions on the actin cytoskeleton and apical cell junctions in the C. elegans spermatheca are influenced by spermathecal anatomy, ovulation state and activation of myosin.Front Cell Dev Biol. 2024 Oct 15;12:1490803. doi: 10.3389/fcell.2024.1490803. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39474353 Free PMC article.
-
Finishing the egg.Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

