Ruthenium-Mediated 18F-Fluorination and Preclinical Evaluation of a New CB1 Receptor Imaging Agent [18F]FPATPP
- PMID: 32479723
- PMCID: PMC7497626
- DOI: 10.1021/acschemneuro.0c00313
Ruthenium-Mediated 18F-Fluorination and Preclinical Evaluation of a New CB1 Receptor Imaging Agent [18F]FPATPP
Abstract
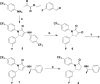
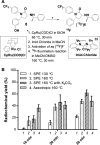
Cannabinoid receptor 1 (CB1R) controls various physiological and pathological conditions, including memory, motivation, and inflammation, and is thus an interesting target for positron emission tomography (PET). Herein, we report a ruthenium-mediated radiolabeling synthesis and preclinical evaluation of a new CB1R specific radiotracer, [18F]FPATPP. [18F]FPATPP was produced with 16.7 ± 5.7% decay-corrected radiochemical yield and >95 GBq/μmol molar activity. The tracer showed high stability, low defluorination, and high specific binding to CB1Rs in mouse brain.
Keywords: CB1R; FPATPP; Positron emission tomography; cannabinoid receptor; fluorine-18; radiofluorination.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
One-step 18F-fluorination of smart positron emission tomography tracer for sensing furin activity in tumors.Nucl Med Biol. 2020 Mar-Apr;82-83:72-79. doi: 10.1016/j.nucmedbio.2020.02.010. Epub 2020 Feb 20. Nucl Med Biol. 2020. PMID: 32109829
-
"In-loop" 18 F-fluorination: A proof-of-concept study.J Labelled Comp Radiopharm. 2019 Jun 15;62(7):292-297. doi: 10.1002/jlcr.3751. J Labelled Comp Radiopharm. 2019. PMID: 31083778
-
Optimization of Direct Aromatic 18F-Labeling of Tetrazines.Molecules. 2022 Jun 22;27(13):4022. doi: 10.3390/molecules27134022. Molecules. 2022. PMID: 35807267 Free PMC article.
-
Electrochemical Radiofluorination of Small Molecules: New Advances.Chem Rec. 2021 Sep;21(9):2397-2410. doi: 10.1002/tcr.202100086. Epub 2021 May 19. Chem Rec. 2021. PMID: 34010479 Review.
-
Aliphatic 18 F-Radiofluorination: Recent Advances in the Labeling of Base-Sensitive Substrates*.ChemMedChem. 2021 Sep 6;16(17):2612-2622. doi: 10.1002/cmdc.202100303. Epub 2021 Aug 4. ChemMedChem. 2021. PMID: 34169672 Review.
Cited by
-
Deoxyfluorination of phenols for chemoselective 18F-labeling of peptides.Nat Protoc. 2023 Nov;18(11):3614-3651. doi: 10.1038/s41596-023-00890-z. Epub 2023 Oct 18. Nat Protoc. 2023. PMID: 37853158 Review.
-
Progress on the application of positron emission tomography imaging of cannabinoid type 1 receptor in neuropsychiatric diseases.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Oct 25;50(5):666-673. doi: 10.3724/zdxbyxb-2021-0063. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34986538 Free PMC article. Review. English.
-
PET molecular imaging for pathophysiological visualization in Alzheimer's disease.Eur J Nucl Med Mol Imaging. 2023 Feb;50(3):765-783. doi: 10.1007/s00259-022-05999-z. Epub 2022 Nov 14. Eur J Nucl Med Mol Imaging. 2023. PMID: 36372804 Free PMC article. Review.
-
Two F-18 radiochemistry methods to synthesize a promising transient receptor potential canonical 5 (TRPC5) radioligand.J Fluor Chem. 2024 Nov;280:110367. doi: 10.1016/j.jfluchem.2024.110367. Epub 2024 Oct 22. J Fluor Chem. 2024. PMID: 40520526
-
Advance and Prospect of Positron Emission Tomography in Alzheimer's disease research.Mol Psychiatry. 2025 Jun 21. doi: 10.1038/s41380-025-03081-2. Online ahead of print. Mol Psychiatry. 2025. PMID: 40544200 Review.
References
-
- Howlett A. C.; Barth F.; Bonner T. I.; Cabral G.; Casellas P.; Devane W. A.; Felder C. C.; Herkenham M.; Mackie K.; Martin B. R.; Mechoulam R.; Pertwee R. G. (2002) International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol Rev. 54 (2), 161–202. 10.1124/pr.54.2.161. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

