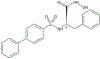
A type IV collagenase inhibitor, N-hydroxy-3-phenyl-2-(4-phenylbenzenesulfonamido) propanamide (BiPS), suppresses skin injury induced by sulfur mustard
- PMID: 32479919
- PMCID: PMC7470515
- DOI: 10.1016/j.taap.2020.115078
A type IV collagenase inhibitor, N-hydroxy-3-phenyl-2-(4-phenylbenzenesulfonamido) propanamide (BiPS), suppresses skin injury induced by sulfur mustard
Abstract
Sulfur mustard (SM) is a highly toxic blistering agent thought to mediate its action, in part, by activating matrix metalloproteinases (MMPs) in the skin and disrupting components of the basement membrane zone (BMZ). Type IV collagenases (MMP-9) degrade type IV collagen in the skin, a major component of the BMZ at the dermal-epidermal junction. In the present studies, a type IV collagenase inhibitor, N-hydroxy-3-phenyl-2-(4-phenylbenzenesulfonamido) propanamide (BiPS), was tested for its ability to protect the skin against injury induced by SM in the mouse ear vesicant model. SM induced inflammation, epidermal hyperplasia and microblistering at the dermal/epidermal junction of mouse ears 24-168 h post-exposure. This was associated with upregulation of MMP-9 mRNA and protein in the skin. Dual immunofluorescence labeling showed increases in MMP-9 in the epidermis and in the adjacent dermal matrix of the SM injured skin, as well as breakdown of type IV collagen in the basement membrane. Pretreatment of the skin with BiPS reduced signs of SM-induced cutaneous toxicity; expression of MMP-9 mRNA and protein was also downregulated in the skin by BiPS. Following BiPS pretreatment, type IV collagen appeared intact and was similar to control skin. These results demonstrate that inhibiting type IV collagenases in the skin improves basement membrane integrity after exposure to SM. BiPS may hold promise as a potential protective agent to mitigate SM induced skin injury.
Keywords: Basement Membrane; MMP-9; Matrix Metalloproteinase Inhibitor; Sulfur Mustard; Type IV Collagen.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there are no conflicts of interest.
Figures

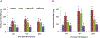
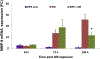






Similar articles
-
Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure.J Appl Toxicol. 2006 May-Jun;26(3):239-46. doi: 10.1002/jat.1134. J Appl Toxicol. 2006. PMID: 16489579
-
Expression of cytokines and chemokines in mouse skin treated with sulfur mustard.Toxicol Appl Pharmacol. 2018 Sep 15;355:52-59. doi: 10.1016/j.taap.2018.06.008. Epub 2018 Jun 20. Toxicol Appl Pharmacol. 2018. PMID: 29935281 Free PMC article.
-
Matrix metalloproteinase-9 expression and release from skin fibroblasts interacting with keratinocytes: Upregulation in response to sulphur mustard.Toxicology. 2009 Sep 1;263(1):26-31. doi: 10.1016/j.tox.2008.08.011. Epub 2008 Sep 3. Toxicology. 2009. PMID: 18809459
-
Acute and chronic effects of sulfur mustard on the skin: a comprehensive review.Cutan Ocul Toxicol. 2010 Dec;29(4):269-77. doi: 10.3109/15569527.2010.511367. Epub 2010 Sep 24. Cutan Ocul Toxicol. 2010. PMID: 20868209 Review.
-
Tissue injury and repair following cutaneous exposure of mice to sulfur mustard.Ann N Y Acad Sci. 2016 Aug;1378(1):118-123. doi: 10.1111/nyas.13125. Epub 2016 Jul 2. Ann N Y Acad Sci. 2016. PMID: 27371823 Free PMC article. Review.
Cited by
-
Skin Models Used to Define Mechanisms of Action of Sulfur Mustard.Disaster Med Public Health Prep. 2023 Oct 18;17:e551. doi: 10.1017/dmp.2023.177. Disaster Med Public Health Prep. 2023. PMID: 37849329 Free PMC article. Review.
References
-
- Amano S, 2016. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp Dermatol 25 Suppl 3, 14–19. - PubMed
-
- Bellon G, Martiny L, Robinet A, 2004. Matrix metalloproteinases and matrikines in angiogenesis. Crit Rev Oncol Hematol 49, 203–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

