The impact of manganese on neurotransmitter systems
- PMID: 32480053
- PMCID: PMC7677177
- DOI: 10.1016/j.jtemb.2020.126554
The impact of manganese on neurotransmitter systems
Abstract
Background: Manganese (Mn) is a metal ubiquitously present in nature and essential for many living organisms. As a trace element, it is required in small amounts for the proper functioning of several important enzymes, and reports of Mn deficiency are indeed rare.
Methods: This mini-review will cover aspects of Mn toxicokinetics and its impact on brain neurotransmission, as well as its Janus-faced effects on humans and other animal's health.
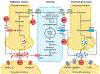
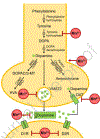
Results: The estimated safe upper limit of intracellular Mn for physiological function is in anarrow range of 20-53 μM.Therefore, intake of higher levels of Mn and the outcomes, especially to the nervous system, have been well documented.
Conclusion: The metal affects mostly the brain by accumulating in specific areas, altering cognitive functions and locomotion, thus severely impacting the health of the exposed organisms.
Keywords: Manganese; Manganism; Neurotoxicity; Trace elements.
Copyright © 2020 Elsevier GmbH. All rights reserved.
Figures
References
-
- Chen P, Bornhorst J, Aschner M, Manganese metabolism in humans, Frontiers in bioscience 23 (2018) 1655–1679. - PubMed
-
- Ávila DS, Gubert P, Roos DH, Puntel RL, Aschner M, Manganese, in: Caballero B (Ed.), Encyclopedia of Food and Health, Elsevier; 2015, pp. 637–641.
-
- Levy BS, Nassetta WJ, Neurologic effects of manganese in humans: a review, International journal of occupational and environmental health 9(2) (2003) 153–63. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

