Efficacy and safety of azithromycin combined with glucocorticoid on refractory Mycoplasma pneumoniae pneumonia in children: A PRISMA-compliant systematic review and meta-analysis
- PMID: 32481378
- PMCID: PMC12245234
- DOI: 10.1097/MD.0000000000020121
Efficacy and safety of azithromycin combined with glucocorticoid on refractory Mycoplasma pneumoniae pneumonia in children: A PRISMA-compliant systematic review and meta-analysis
Abstract
Introduction: The aim of this study was to evaluate the efficacy and safety of azithromycin (AZI) combined with glucocorticoid (GC) in the treatment of children with refractory Mycoplasma pneumoniae.
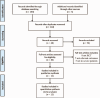
Methods: Computer search for PubMed, EMbase, Cochrane Library, China Biomedical Literature Database (CBMdisc), China Knowledge Network (CNKI), Wanfang, VIP (VIP), and a randomized controlled trial (RCT) of AZI combined with GC in the treatment of children with refractory Mycoplasma pneumoniae pneumonia test (RCT), the search time limit is built until March 20, 2019. Two researchers independently performed literature screening, data extraction, and literature risk bias, and meta-analysis was performed using RevMan 5.3 software.
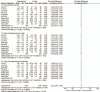
Results: A total of 12 RCTs were included, including 1130 patients. Meta-analysis showed that AZI combined with GC therapy significantly improved the total effective rate of the disease compared with the conventional treatment group (odds ratio [OR] = 6.37; 95% confidence interval [CI] 4.03, 10.07; P < .00001; I = 0%), effectively shortened the antipyretic time (SMD = -2.29; 95% CI -2.70, -1.88; P < .0001); promoted lung inflammation absorption (SMD = -1.89; 95% CI -2.38, -1.40; P < .0001), reduced cough time (SMD = -2.39; 95% CI -2.80, -1.99; P < .0001); shortened hospital stay (SMD = -2.19; 95% CI -3.21, -1.17; P < .0001); improved imaging findings (OR = 5.38; 95% CI 1.09, 26.51, P = .04); reduced inflammation index (SMD = -3.15; 95% CI -4.93, -1.36; P = .004); improved immune function (SMD = 1.29; 95% CI -0.02, 2.60; P < .0001); had no significant adverse reactions (OR = 1.18; 95% CI 0.71, 1.98; P = .53).
Conclusions: According to the current limited research evidence, the addition of GCs to the conventional treatment of refractory Mycoplasma pneumoniae in children can improve the clinical efficacy to a certain extent, and the safety is better. However, due to the quality and quantity of the included literature, the conclusions of this study need to be confirmed by more high-quality studies.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Figures





References
-
- Respiratory group. pediatrics branch of Chinese medical association. Guidelines for the management of community-acquired pneumonia in children (2013revision) (China). Chin j Pediatr 2013;51:856–62.
-
- Wang J, Sun J, Gao CL, et al. The effect of fiberoptic bronchoscopy and bronchoalveolar lavage on refractory mycoplasma pneumoniae pneumonia in children (China). J Clin Pediatr 2017;35:16–8.
-
- Cao LF. The present state and development for the diagnosis and treatment of refractory Pneumoniae pneumonia in children (China). J Clin Pediatr 2010;28:94–7.
-
- Bu XF, Kong ZY, Wang J. Pathogenesis and progress of diagnosis and treatment of refractory mycoplasma pneumonia in children (China). Guangdong Medical Journal 2018;39:295–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

