Epigenetic Factors That Control Pericentric Heterochromatin Organization in Mammals
- PMID: 32481609
- PMCID: PMC7349813
- DOI: 10.3390/genes11060595
Epigenetic Factors That Control Pericentric Heterochromatin Organization in Mammals
Abstract
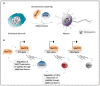
Pericentric heterochromatin (PCH) is a particular form of constitutive heterochromatin that is localized to both sides of centromeres and that forms silent compartments enriched in repressive marks. These genomic regions contain species-specific repetitive satellite DNA that differs in terms of nucleotide sequences and repeat lengths. In spite of this sequence diversity, PCH is involved in many biological phenomena that are conserved among species, including centromere function, the preservation of genome integrity, the suppression of spurious recombination during meiosis, and the organization of genomic silent compartments in the nucleus. PCH organization and maintenance of its repressive state is tightly regulated by a plethora of factors, including enzymes (e.g., DNA methyltransferases, histone deacetylases, and histone methyltransferases), DNA and histone methylation binding factors (e.g., MECP2 and HP1), chromatin remodeling proteins (e.g., ATRX and DAXX), and non-coding RNAs. This evidence helps us to understand how PCH organization is crucial for genome integrity. It then follows that alterations to the molecular signature of PCH might contribute to the onset of many genetic pathologies and to cancer progression. Here, we describe the most recent updates on the molecular mechanisms known to underlie PCH organization and function.
Keywords: ATRX; DNA methylation; HP1; MeCP2; non-coding RNAs; pericentric heterochromatin; repressive compartments; satellite DNA.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

