Valosin-Containing Protein, a Calcium-Associated ATPase Protein, in Endoplasmic Reticulum and Mitochondrial Function and Its Implications for Diseases
- PMID: 32481679
- PMCID: PMC7312078
- DOI: 10.3390/ijms21113842
Valosin-Containing Protein, a Calcium-Associated ATPase Protein, in Endoplasmic Reticulum and Mitochondrial Function and Its Implications for Diseases
Abstract
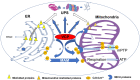
Endoplasmic reticulum (ER) and mitochondrion are the key organelles in mammal cells and play crucial roles in a variety of biological functions in both physiological and pathological conditions. Valosin-containing protein (VCP), a newly identified calcium-associated ATPase protein, has been found to be involved in both ER and mitochondrial function. Impairment of VCP, caused by structural mutations or alterations of expressions, contributes to the development of various diseases, through an integrating effect on ER, mitochondria and the ubiquitin-proteasome system, by interfering with protein degradation, subcellular translocation and calcium homeostasis. Thus, understanding the role and the molecular mechanisms of VCP in these organelles brings new insights to the pathogenesis of the associated diseases, and leads to the discovery of new therapeutic strategies. In this review, we summarized the progress of studies on VCP, in terms of its regulation of ER and mitochondrial function and its implications for the associated diseases, focusing on the cancers, heart disease, and neurodegenerative disorders.
Keywords: calcium homeostasis; disease; endoplasmic reticulum; mitochondria; valosin-containing protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

