Products of Lipid Peroxidation as a Factor in the Toxic Effect of Silver Nanoparticles
- PMID: 32481688
- PMCID: PMC7321096
- DOI: 10.3390/ma13112460
Products of Lipid Peroxidation as a Factor in the Toxic Effect of Silver Nanoparticles
Abstract
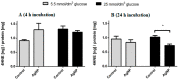
In our previous study we have shown that nanoparticles have different effects depending on the energy metabolism of the cell, which is an important factor in the context of oncology and diabetes. Here we assess the influence of AgNPs on cellular lipid components in varying glucose concentrations. To assess the effect of silver nanoparticles on cell lipids, we measured cell viability, the fluidity of the cell membranes, the content of amino groups in proteins, the level of lipid peroxidation products, the concentration of 4-hydroxynonenal (4-HNE), and the concentration of lipid peroxides. The obtained results show differences in the formation of lipid peroxidation products in cells exposed to oxidative stress induced by nanoparticles. In addition, we have shown that the metabolic state of the cell is a factor significantly affecting this process.
Keywords: lipid peroxidation; oxidative stress; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

