Cavitary tuberculosis: the gateway of disease transmission
- PMID: 32482293
- PMCID: PMC7357333
- DOI: 10.1016/S1473-3099(20)30148-1
Cavitary tuberculosis: the gateway of disease transmission
Abstract
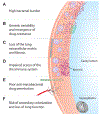
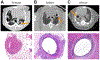
Tuberculosis continues to be a major threat to global health. Cavitation is a dangerous consequence of pulmonary tuberculosis associated with poor outcomes, treatment relapse, higher transmission rates, and development of drug resistance. However, in the antibiotic era, cavities are often identified as the most extreme outcome of treatment failure and are one of the least-studied aspects of tuberculosis. We review the epidemiology, clinical features, and concurrent standards of care for individuals with cavitary tuberculosis. We also discuss developments in the understanding of tuberculosis cavities as dynamic physical and biochemical structures that interface the host response with a unique mycobacterial niche to drive tuberculosis-associated morbidity and transmission. Advances in preclinical models and non-invasive imaging can provide valuable insights into the drivers of cavitation. These insights will guide the development of specific pharmacological interventions to prevent cavitation and improve lung function for individuals with tuberculosis.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare no conflicts of interest.
Figures
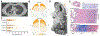

References
-
- Global tuberculosis report 2019. Geneva: World Health Organization, 2019.
-
- Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002; 360(9332): 528–34. - PubMed
-
- Dannenberg AM Jr. Pathogenesis of human pulmonary tuberculosis: insights from the rabbit model. Washington: ASM Press; 2006.
-
- Canetti G The tubercle bacillus in the pulmonary lesion of man: histobacteriology and its bearing on the therapy of pulmonary tuberculosis: Springer Publishing Company; 1955.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

