Molecular design of a signaling system influences noise in protein abundance under acid stress in different γ-Proteobacteria
- PMID: 32482722
- PMCID: PMC8404709
- DOI: 10.1128/JB.00121-20
Molecular design of a signaling system influences noise in protein abundance under acid stress in different γ-Proteobacteria
Abstract
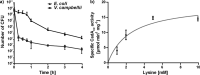
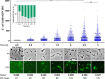
Bacteria have evolved different signaling systems to sense and adapt to acid stress. One of these systems, the CadABC-system, responds to a combination of low pH and lysine availability. In Escherichia coli, the two signals are sensed by the pH sensor and transcription activator CadC and the co-sensor LysP, a lysine-specific transporter. Activated CadC promotes the transcription of the cadBA operon, which codes for the lysine decarboxylase CadA and the lysine/cadaverine antiporter CadB. The copy number of CadC is controlled translationally. Using a bioinformatics approach, we identified the presence of CadC with ribosomal stalling motifs together with LysP in species of the Enterobacteriaceae family. In contrast, we identified CadC without stalling motifs in species of the Vibrionaceae family, but the LysP co-sensor was not identified. Therefore, we compared the output of the Cad system in single cells of the distantly related organisms E. coli and V. campbellii using fluorescently-tagged CadB as the reporter. We observed a heterogeneous output in E. coli, and all the V. campbellii cells produced CadB. The copy number of the pH sensor CadC in E. coli was extremely low (≤4 molecules per cell), but it was 10-fold higher in V. campbellii An increase in the CadC copy number in E. coli correlated with a decrease in heterogeneous behavior. This study demonstrated how small changes in the design of a signaling system allow a homogeneous output and, thus, adaptation of Vibrio species that rely on the CadABC-system as the only acid resistance system.Importance Acid resistance is an important property of bacteria, such as Escherichia coli, to survive acidic environments like the human gastrointestinal tract. E. coli possess both passive and inducible acid resistance systems to counteract acidic environments. Thus, E. coli evolved sophisticated signaling systems to sense and appropriately respond to environmental acidic stress by regulating the activity of its three inducible acid resistance systems. One of these systems is the Cad system that is only induced under moderate acidic stress in a lysine-rich environment by the pH-responsive transcriptional regulator CadC. The significance of our research is in identifying the molecular design of the Cad systems in different Proteobacteria and their target expression noise at single cell level during acid stress conditions.
Copyright © 2020 American Society for Microbiology.
Figures





References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

