MYC protein stability is negatively regulated by BRD4
- PMID: 32482868
- PMCID: PMC7306749
- DOI: 10.1073/pnas.1919507117
MYC protein stability is negatively regulated by BRD4
Abstract
The protooncogene MYC regulates a variety of cellular processes, including proliferation and metabolism. Maintaining MYC at homeostatic levels is critical to normal cell function; overexpression drives many cancers. MYC stability is regulated through phosphorylation: phosphorylation at Thr58 signals degradation while Ser62 phosphorylation leads to its stabilization and functional activation. The bromodomain protein 4 (BRD4) is a transcriptional and epigenetic regulator with intrinsic kinase and histone acetyltransferase (HAT) activities that activates transcription of key protooncogenes, including MYC We report that BRD4 phosphorylates MYC at Thr58, leading to MYC ubiquitination and degradation, thereby regulating MYC target genes. Importantly, BRD4 degradation, but not inhibition, results in increased levels of MYC protein. Conversely, MYC inhibits BRD4's HAT activity, suggesting that MYC regulates its own transcription by limiting BRD4-mediated chromatin remodeling of its locus. The MYC stabilizing kinase, ERK1, regulates MYC levels directly and indirectly by inhibiting BRD4 kinase activity. These findings demonstrate that BRD4 negatively regulates MYC levels, which is counteracted by ERK1 activation.
Keywords: BRD4 histone acetyltransferase; BRD4 kinase; ERK1; MYC phosphorylation; MYC stability.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


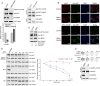
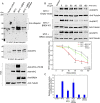
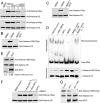

References
-
- Bretones G., Delgado M. D., León J., Myc and cell cycle control. Biochim. Biophys. Acta 1849, 506–516 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

