Exosomal miR-130b-3p targets SIK1 to inhibit medulloblastoma tumorigenesis
- PMID: 32483145
- PMCID: PMC7264172
- DOI: 10.1038/s41419-020-2621-y
Exosomal miR-130b-3p targets SIK1 to inhibit medulloblastoma tumorigenesis
Abstract
Exosomes are an important carrier for cell communication. miRNAs in exosomes are potential biomarkers and therapeutic targets in different types of cancer. However, the role of exosomal miRNAs in medulloblastoma (MB) patients is largely unknown. In this study, we reported that there was a higher level of miR-130b-3p in exosomes derived from MB patient plasma compared with exosomes from healthy control plasma. Exosomes from MB patient plasma could transfer miR-130b-3p to an MB cell line and played suppressor roles for cell proliferation. miR-130b-3p suppressed MB tumorigenesis by targeting a previously unknown target, serine/threonine-protein kinase 1 (SIK1), through the p53 signaling pathways. In addition, we found an unreported role of SIK1 in promoting MB tumor growth and an SIK1 inhibitor could inhibit MB cell proliferation. This research provides new insight into the molecular mechanism of MB and may provide a new therapeutic strategy for MB treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
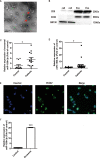

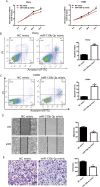
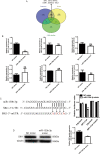
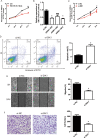



References
-
- Ning MS, Perkins SM, Dewees T, Shinohara ET. Evidence of high mortality in long term survivors of childhood medulloblastoma. J. Neuro-Oncol. 2015;122:321–3-27. - PubMed
-
- Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. - PubMed
-
- Gajjar A, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. - PubMed
-
- Rutkowski S, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J. Clin. Oncol. 2010;28:4961–4968. - PubMed
-
- Ellison DW, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J. Clin. Oncol. 2005;23:7951–7957. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

