Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-Drug Conjugates (ADCs)
- PMID: 32483228
- PMCID: PMC7264231
- DOI: 10.1038/s41598-020-65860-x
Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-Drug Conjugates (ADCs)
Abstract
Antibody-Drug Conjugates (ADCs) developed as a targeted treatment approach to deliver toxins directly to cancer cells are one of the fastest growing classes of oncology therapeutics, with eight ADCs and two immunotoxins approved for clinical use. However, selection of an optimum target and payload combination, to achieve maximal therapeutic efficacy without excessive toxicity, presents a significant challenge. We have developed a platform to facilitate rapid and cost-effective screening of antibody and toxin combinations for activity and safety, based on streptavidin-biotin conjugation. For antibody selection, we evaluated internalization by target cells using streptavidin-linked antibodies conjugated to biotinylated saporin, a toxin unable to cross cell membranes. For payload selection, we biotinylated toxins and conjugated them to antibodies linked to streptavidin to evaluate antitumour activity and pre-clinical safety. As proof of principle, we compared trastuzumab conjugated to emtansine via streptavidin-biotin (Trastuzumab-SB-DM1) to the clinically approved trastuzumab emtansine (T-DM1). We showed comparable potency in reduction of breast cancer cell survival in vitro and in growth restriction of orthotopic breast cancer xenografts in vivo. Our findings indicate efficient generation of functionally active ADCs. This approach can facilitate the study of antibody and payload combinations for selection of promising candidates for future ADC development.
Conflict of interest statement
S.N.K. and J.F.S. are founders and shareholders of Epsilogen Ltd. DET is co-founder of Femtogenix Ltd. All other authors declare no conflicts of interest.
Figures


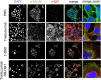
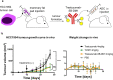

References
-
- Bross PF, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001;7:1490–1496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

