Exosome-like vesicles derived from Hertwig's epithelial root sheath cells promote the regeneration of dentin-pulp tissue
- PMID: 32483427
- PMCID: PMC7254987
- DOI: 10.7150/thno.43156
Exosome-like vesicles derived from Hertwig's epithelial root sheath cells promote the regeneration of dentin-pulp tissue
Abstract
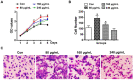
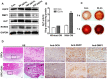
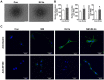
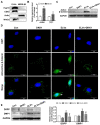
Background: The formation of dentin-pulp involves complex epithelial-mesenchymal interactions between Hertwig's epithelial root sheath cells (HERS) and dental papilla cells (DPCs). Earlier studies have identified some of the regulatory molecules participating in the crosstalk between HERS and DPCs and the formation of dentin-pulp. In the present study we focused on the role of HERS-secreted exosomes in DPCs and the formation of dentin-pulp. Specifically, we hypothesized that exosome-like vesicles (ELVs) might mediate the function of HERS and trigger lineage-specific differentiation of dental mesenchymal cells. To test our hypothesis, we evaluated the potential of ELVs derived from a HERS cell line (ELVs-H1) in inducing in vitro and in vivo differentiation of DPCs. Methods: ELVs-H1 were characterized using transmission electron microscopy and dynamic light scattering. The proliferation, migration, and odontoblast differentiation of DPCs after treatment with ELVs-H1, was detected by CCK8, transwell, ALP, and mineralization assays, respectively. Real time PCR and western blotting were used to detect gene and protein expression. For in vivo studies, DPC cells were mixed with collagen gel combined with or without ELVs and transplanted into the renal capsule of rats or subcutaneously into nude mice. HE staining and immunostaining were used to verify the regeneration of dentin-pulp and expression of odontoblast differentiation markers. Results: ELVs-H1 promoted the migration and proliferation of DPCs and also induced odontogenic differentiation and activation of Wnt/β-catenin signaling. ELVs-H1 also contributed to tube formation and neural differentiation in vitro. In addition, ELVs-H1 attached to the collagen gel, and were slowly released and endocytosed by DPCs, enhancing cell survival. ELVs-H1 together with DPCs triggered regeneration of dental pulp-dentin like tissue comprised of hard (reparative dentin-like tissue) and soft (blood vessels and neurons) tissue, in an in vivo tooth root slice model. Conclusion: Our data highlighted the potential of ELVs-H1 as biomimetic tools in providing a microenvironment for specific differentiation of dental mesenchymal stem cells. From a developmental perspective, these vesicles might be considered as novel mediators facilitating the epithelial-mesenchymal crosstalk. Their instructive potency might be exploited for the regeneration of dental pulp-dentin tissues.
Keywords: Hertwig's epithelial root sheath cell; epithelial-mesenchymal interaction; exosome-like vesicle; odontogenic differentiation; pulp-dentin regeneration..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–32. - PubMed
-
- Morito A, Kida Y, Suzuki K, Inoue K, Kuroda N, Gomi K. et al. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol. 2009;72:51–64. - PubMed
-
- Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res. 2018;97:1137–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

