CDK12 and PAK2 as novel therapeutic targets for human gastric cancer
- PMID: 32483448
- PMCID: PMC7255043
- DOI: 10.7150/thno.46137
CDK12 and PAK2 as novel therapeutic targets for human gastric cancer
Abstract
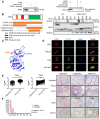
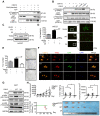
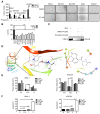
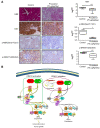
Background: Gastric cancer remains the second leading cause of cancer-related death, and the third in mortality due to lack of effective therapeutic targets for late stage cancer patients. This study aims to identify potential druggable target biomarkers as potential therapeutic options for patients with gastric cancer. Methods: Immunohistochemistry of human gastric tumor tissues was conducted to determine the expression level of cyclin-dependent kinase 12 (CDK12). Multiple in vitro and in vivo assays such as RNAi, mass spectrometry, computer docking models, kinase assays, cell xenograft NU/NU mouse models (CDXs) and patient-derived xenograft NOD/SCID mouse models (PDXs) were conducted to study the function and molecular interaction of CDK12 with p21 activated kinase 2 (PAK2), as well as to find CDK12 inhibitors as potential treatment options for human gastric cancer. Results: Here we identified that CDK12 is a driver gene in human gastric cancer growth. Mechanistically, CDK12 directly binds to and phosphorylates PAK2 at T134/T169 to activate MAPK signaling pathway. We further identified FDA approved clinical drug procaterol can serve as an effective CDK12 inhibitor, leading to dramatic restriction of cancer cell proliferation and tumor growth in human gastric cancer cells and PDXs. Conclusions: Our data highlight the potential of CDK12/PAK2 as therapeutic targets for patients with gastric cancer, and we propose procaterol treatment as a novel therapeutic strategy for human gastric cancer.
Keywords: CDK12; PAK2; gastric cancer; phosphorylation; procaterol.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Activation of P21-activated protein kinase 2 is an independent prognostic predictor for patients with gastric cancer.Diagn Pathol. 2014 Mar 12;9:55. doi: 10.1186/1746-1596-9-55. Diagn Pathol. 2014. PMID: 24621074 Free PMC article.
-
Expression pattern of CDK12 protein in gastric cancer and its positive correlation with CD8+ cell density and CCL12 expression.Int J Med Sci. 2019 Aug 6;16(8):1142-1148. doi: 10.7150/ijms.34541. eCollection 2019. Int J Med Sci. 2019. PMID: 31523177 Free PMC article.
-
Development of an orally bioavailable CDK12/13 degrader and induction of synthetic lethality with AKT pathway inhibition.Cell Rep Med. 2024 Oct 15;5(10):101752. doi: 10.1016/j.xcrm.2024.101752. Epub 2024 Sep 30. Cell Rep Med. 2024. PMID: 39353441 Free PMC article.
-
CDK12: an emerging therapeutic target for cancer.J Clin Pathol. 2018 Nov;71(11):957-962. doi: 10.1136/jclinpath-2018-205356. Epub 2018 Aug 13. J Clin Pathol. 2018. PMID: 30104286 Free PMC article. Review.
-
Gene expression regulation by CDK12: a versatile kinase in cancer with functions beyond CTD phosphorylation.Exp Mol Med. 2020 May;52(5):762-771. doi: 10.1038/s12276-020-0442-9. Epub 2020 May 25. Exp Mol Med. 2020. PMID: 32451425 Free PMC article. Review.
Cited by
-
CDK12 is a potential biomarker for diagnosis, prognosis and immunomodulation in pan-cancer.Sci Rep. 2024 Mar 19;14(1):6574. doi: 10.1038/s41598-024-56831-7. Sci Rep. 2024. PMID: 38503865 Free PMC article.
-
Cyclin-dependent kinase 12 deficiency reprogrammes cellular metabolism to alleviate ferroptosis potential and promote the progression of castration-resistant prostate cancer.Clin Transl Med. 2024 May;14(5):e1678. doi: 10.1002/ctm2.1678. Clin Transl Med. 2024. PMID: 38736108 Free PMC article.
-
The mechanism of BUD13 m6A methylation mediated MBNL1-phosphorylation by CDK12 regulating the vasculogenic mimicry in glioblastoma cells.Cell Death Dis. 2022 Dec 3;13(12):1017. doi: 10.1038/s41419-022-05426-z. Cell Death Dis. 2022. PMID: 36463205 Free PMC article.
-
Long intergenic noncoding RNA 00665 promotes proliferation and inhibits apoptosis in colorectal cancer by regulating miR-126-5p.Aging (Albany NY). 2021 Apr 20;13(10):13571-13584. doi: 10.18632/aging.202874. Epub 2021 Apr 20. Aging (Albany NY). 2021. PMID: 33878735 Free PMC article.
-
RNA-binding protein MEX3D promotes cervical carcinoma tumorigenesis by destabilizing TSC22D1 mRNA.Cell Death Discov. 2022 May 5;8(1):250. doi: 10.1038/s41420-022-01049-7. Cell Death Discov. 2022. PMID: 35513372 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

