Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19
- PMID: 32483554
- PMCID: PMC7202249
- DOI: 10.1021/acscentsci.0c00489
Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19
Erratum in
-
Correction to Remdesivir: A Review of Its Discovery and Development Leading to Human Clinical Trials for Treatment of COVID-19.ACS Cent Sci. 2020 Jun 24;6(6):1009. doi: 10.1021/acscentsci.0c00747. Epub 2020 Jun 16. ACS Cent Sci. 2020. PMID: 32607448 Free PMC article.
Abstract
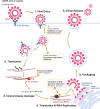
The global pandemic of SARS-CoV-2, the causative viral pathogen of COVID-19, has driven the biomedical community to action-to uncover and develop antiviral interventions. One potential therapeutic approach currently being evaluated in numerous clinical trials is the agent remdesivir, which has endured a long and winding developmental path. Remdesivir is a nucleotide analogue prodrug that perturbs viral replication, originally evaluated in clinical trials to thwart the Ebola outbreak in 2014. Subsequent evaluation by numerous virology laboratories demonstrated the ability of remdesivir to inhibit coronavirus replication, including SARS-CoV-2. Here, we provide an overview of remdesivir's discovery, mechanism of action, and the current studies exploring its clinical effectiveness.
Copyright © 2020 U.S. Government.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Fields B. N.; Knipe D. M.; Howley P. M.. Coronavirus. In Fields Virology, 6th ed.; Wolters Kluwer Health/Lippincott Williams and Wilkins, 2013; pp 825–858.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

