Clonal Evolution and Heterogeneity of Osimertinib Acquired Resistance Mechanisms in EGFR Mutant Lung Cancer
- PMID: 32483558
- PMCID: PMC7263628
- DOI: 10.1016/j.xcrm.2020.100007
Clonal Evolution and Heterogeneity of Osimertinib Acquired Resistance Mechanisms in EGFR Mutant Lung Cancer
Abstract
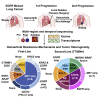
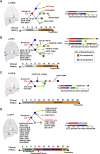
Clonal evolution of osimertinib-resistance mechanisms in EGFR mutant lung adenocarcinoma is poorly understood. Using multi-region whole-exome and RNA sequencing of prospectively collected pre- and post-osimertinib-resistant tumors, including at rapid autopsies, we identify a likely mechanism driving osimertinib resistance in all patients analyzed. The majority of patients acquire two or more resistance mechanisms either concurrently or in temporal sequence. Focal copy-number amplifications occur subclonally and are spatially and temporally separated from common resistance mutations such as EGFR C797S. MET amplification occurs in 66% (n = 6/9) of first-line osimertinib-treated patients, albeit spatially heterogeneous, often co-occurs with additional acquired focal copy-number amplifications and is associated with early progression. Noteworthy osimertinib-resistance mechanisms discovered include neuroendocrine differentiation without histologic transformation, PD-L1, KRAS amplification, and ESR1-AKAP12, MKRN1-BRAF fusions. The subclonal co-occurrence of acquired genomic alterations upon osimertinib resistance will likely require targeting multiple resistance mechanisms by combination therapies.
Trial registration: ClinicalTrials.gov NCT02759835.
Conflict of interest statement
C.K. receives research funding to his current institution (Georgetown University) from AstraZeneca, Novartis, and Tesaro and received one-time travel support from CARIS Life Science. C.T. owns stock in Gilead, Celgene, Exelixis, Clovis, and Trevena, and he is an employee of the Food and Drug Administration outside of the submitted work. Z.P. has served as a compensated consultant or received honoraria from AstraZeneca, Spectrum, Ariad/Takeda, Novartis, ImmunoGen, AbbVie, GuardantHealth, Genentech, Eli Lilly, InCyte, and Medtronic and receives institutional research funding from Novartis, Takeda, Spectrum, AstraZeneca, and Tesaro. U.G. has a clinical trial agreement (CTA) with AstraZeneca for the current study and receives research funding from AstraZeneca, Esanex, and Aurigene. U.G. is currently an employee of Bristol Myers Squibb. The other authors have no conflicts of interest to report.
Figures






References
-
- Oxnard G.R., Hu Y., Mileham K.F., Husain H., Costa D.B., Tracy P., Feeney N., Sholl L.M., Dahlberg S.E., Redig A.J. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018;4:1527–1534. - PMC - PubMed
-
- Le X., Puri S., Negrao M.V., Nilsson M.B., Robichaux J., Boyle T., Hicks J.K., Lovinger K.L., Roarty E., Rinsurongkawong W. Landscape of EGFR -dependent and -independent resistance mechanisms to osimertinib and continuation therapy post-progression in EGFR-mutant NSCLC. Clin. Cancer Res. 2018;24:6195–6203. - PMC - PubMed
-
- Yang Z., Yang N., Ou Q., Xiang Y., Jiang T., Wu X., Bao H., Tong X., Wang X., Shao Y.W. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018;24:3097–3107. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

