Yap1-Scribble polarization is required for hematopoietic stem cell division and fate
- PMID: 32483624
- PMCID: PMC7568035
- DOI: 10.1182/blood.2019004113
Yap1-Scribble polarization is required for hematopoietic stem cell division and fate
Abstract
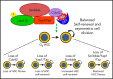
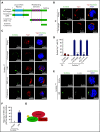
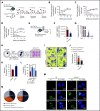
Yap1 and its paralogue Taz largely control epithelial tissue growth. We have identified that hematopoietic stem cell (HSC) fitness response to stress depends on Yap1 and Taz. Deletion of Yap1 and Taz induces a loss of HSC quiescence, symmetric self-renewal ability, and renders HSC more vulnerable to serial myeloablative 5-fluorouracil treatment. This effect depends on the predominant cytosolic polarization of Yap1 through a PDZ domain-mediated interaction with the scaffold Scribble. Scribble and Yap1 coordinate to control cytoplasmic Cdc42 activity and HSC fate determination in vivo. Deletion of Scribble disrupts Yap1 copolarization with Cdc42 and decreases Cdc42 activity, resulting in increased self-renewing HSC with competitive reconstitution advantages. These data suggest that Scribble/Yap1 copolarization is indispensable for Cdc42-dependent activity on HSC asymmetric division and fate. The combined loss of Scribble, Yap1, and Taz results in transcriptional upregulation of Rac-specific guanine nucleotide exchange factors, Rac activation, and HSC fitness restoration. Scribble links Cdc42 and the cytosolic functions of the Hippo signaling cascade in HSC fate determination.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

