Clinical features of LRP4/agrin-antibody-positive myasthenia gravis: A multicenter study
- PMID: 32483837
- PMCID: PMC7496236
- DOI: 10.1002/mus.26985
Clinical features of LRP4/agrin-antibody-positive myasthenia gravis: A multicenter study
Abstract
Introduction: Our aim in this study was to identify the prevalence and clinical characteristics of LRP4/agrin-antibody-positive double-seronegative myasthenia gravis (DNMG).
Methods: DNMG patients at 16 sites in the United States were tested for LRP4 and agrin antibodies, and the clinical data were collected.
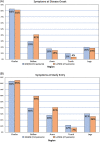
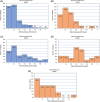
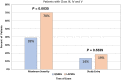
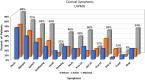
Results: Of 181 DNMG patients, 27 (14.9%) were positive for either low-density lipoprotein receptor-related protein 4 (LRP4) or agrin antibodies. Twenty-three DNMG patients (12.7%) were positive for both antibodies. More antibody-positive patients presented with generalized symptoms (69%) compared with antibody-negative patients (43%) (P ≤ .02). Antibody-positive patients' maximum classification on the Myasthenia Gravis Foundation of America (MGFA) scale was significantly higher than that for antibody-negative patients (P ≤ .005). Seventy percent of antibody-positive patients were classified as MGFA class III, IV, or V compared with 39% of antibody-negative patients. Most LRP4- and agrin-antibody-positive patients (24 of 27, 89%) developed generalized myathenia gravis (MG), but with standard MG treatment 81.5% (22 of 27) improved to MGFA class I or II during a mean follow-up of 11 years.
Discussion: Antibody-positive patients had more severe clinical disease than antibody-negative patients. Most DNMG patients responded to standard therapy regardless of antibody status.
Keywords: LRP4; agrin; clinical features; myasthenia gravis; neuromuscular transmission disorders; seronegative myasthenia gravis.
© 2020 The Authors. Muscle & Nerve published by Wiley Periodicals, Inc.
Conflict of interest statement
M.H.R.: consultant and research support from Alexion and Allergan; research support from UCB, Mallinckrodt, Cytokinetics, Momenta, Shire Takeda, Orion, Biohaven, Catalyst, Grifols, Seikagaku, and Ra Pharmaceuticals. B.M.Q.: research support from Alexion, UCB, Mallinckrodt, Cytokinetics, Momenta, Shire Takeda, Orion, Biohaven, Catalyst, Grifols, Seikagaku, and Ra Pharmaceuticals. J.F.H.: research support from Alexion, Argenx BVBA, Centers for Disease Control and Prevention, Muscular Dystrophy Association (MDA), National Institutes of Health (NIH, including National Institute of Neurological Disorders and Stroke and National Institute of Arthritis and Musculoskeletal and Skin Diseases), and Ra Pharmaceuticals; honoraria from Alexion; and nonfinancial support from Alexion, Argenx BVBA, Ra Pharmaceuticals, and Toleranzia. M.M.D.: consultant for ArgenX, CSL‐Behring, Kezar, Momenta, NuFactor, RMS Medical, Sanofi Genzyme, Shire Takeda, and Spark; grants from Alexion, Alnylam, Amicus, Biomarin, Bristol‐Myers Squibb, Catalyst, CSL‐Behring, US Food and Drug Administration/Office of Orphan Products Development, GlaxoSmithKline, Genentech, Grifols, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Sanofi Genzyme, Octapharma, Orphazyme, Sarepta, Shire Takeda, Spark, UCB Biopharma, Viromed, and TMA. C.J.: speaker's bureau for Mitsubishi Tanabe Pharma America, Avanir, CSL Behring, and Strongbridge; research support and consultant fees from Cytokinetics and Mitsubishi Tanabe Pharma America; consultant fees from Argenx, Alexion, and ITF; data safety monitoring board for Mallinckrodt, Brainstorm, and Anelixis. T.V.: speaker and/or consultant for Alexion, ARGX, and UCB; participated in clinical trials sponsored by Alexion, ARGX, Ra Pharmaceuticals, UCB, and Grifols. G.S.: consultant for Alexion. R.P.L.: over past 2 years has been funded for research support from the NIH, National Multiple Sclerosis Society (USA), Genentech, Teva, Novartis, Medimmune, Ra Pharmaceuticals, Argenx, Alexion, Catalyst, and Chugai; served as a consultant to Argenx, Novartis, Mallinckrodt, Catalyst, GLG, Alpha Sites, Insights Consulting, Informa Pharma Consulting, Slingshot Consulting, Schlessinger Group Consulting, Health Choices, Adivo Associates, Haven Consulting, kc2 Medical Communications, and Cello Health Bioconsulting. I.L.: funding support from the Myasthenia Gravis Foundation of America (MGFA) for the MG Registry project. R.J.N.: research support from the NIH, Genentech, Alexion, Ra Pharmaceuticals, MGFA, Momenta, Argenx, Annexon Biosciences, and Grifols; consultant/advisor for Alexion Pharmaceuticals, Argenx, CSL Behring, Grifols, Ra Pharmaceuticals, Roivant, Viela Bio, and Momenta (no conflicts directly related to this study). J.A.F.: funding from Mallinckrodt Pharmaceuticalsand consultant fees from Biogen. Z.S.: research support from Cytokinetics, Mallinckrodt, and Biohaven; consulting fees from Biohaven; payment from Wiley for his position as editor‐in‐chief for
Figures
References
-
- Hoch WM, Helms J, Newsom‐Davis S, Melms J, Vincent A. Auto‐antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365‐368. - PubMed
-
- Sanders DB, El‐Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60:1978‐1980. - PubMed
-
- Birmanns B, Brenner T, Abramsky O, Steiner I. Seronegative myasthenia gravis: clinical features, response to therapy and synthesis of acetylcholine receptor antibodies in vitro. J Neurol Sci. 1991;102:184‐189. - PubMed
-
- Li L, Xiong WC, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159‐188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

