PD-1 blockade inhibits osteoclast formation and murine bone cancer pain
- PMID: 32484460
- PMCID: PMC7324182
- DOI: 10.1172/JCI133334
PD-1 blockade inhibits osteoclast formation and murine bone cancer pain
Abstract
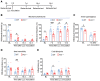
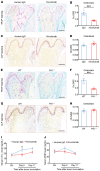
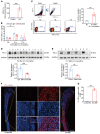
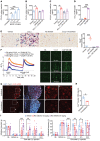
Emerging immune therapy, such as with the anti-programmed cell death-1 (anti-PD-1) monoclonal antibody nivolumab, has shown efficacy in tumor suppression. Patients with terminal cancer suffer from cancer pain as a result of bone metastasis and bone destruction, but how PD-1 blockade affects bone cancer pain remains unknown. Here, we report that mice lacking Pdcd1 (Pd1-/-) demonstrated remarkable protection against bone destruction induced by femoral inoculation of Lewis lung cancer cells. Compared with WT mice, Pd1-/- mice exhibited increased baseline pain sensitivity, but the development of bone cancer pain was compromised in Pd1-/- mice. Consistently, these beneficial effects in Pd1-/- mice were recapitulated by repeated i.v. applications of nivolumab in WT mice, even though nivolumab initially increased mechanical and thermal pain. Notably, PD-1 deficiency or nivolumab treatment inhibited osteoclastogenesis without altering tumor burden. PD-L1 and CCL2 are upregulated within the local tumor microenvironment, and PD-L1 promoted RANKL-induced osteoclastogenesis through JNK activation and CCL2 secretion. Bone cancer upregulated CCR2 in primary sensory neurons, and CCR2 antagonism effectively reduced bone cancer pain. Our findings suggest that, despite a transient increase in pain sensitivity following each treatment, anti-PD-1 immunotherapy could produce long-term benefits in preventing bone destruction and alleviating bone cancer pain by suppressing osteoclastogenesis.
Keywords: Bone disease; Cancer; Cell Biology; Neuroscience; Pain.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

