Bruton's tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis
- PMID: 32484802
- PMCID: PMC7456252
- DOI: 10.1172/JCI138448
Bruton's tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis
Abstract
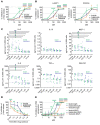
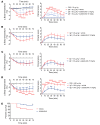
No known therapies can prevent anaphylaxis. Bruton's tyrosine kinase (BTK) is an enzyme thought to be essential for high-affinity IgE receptor (FcεRI) signaling in human cells. We tested the hypothesis that FDA-approved BTK inhibitors (BTKis) would prevent IgE-mediated responses including anaphylaxis. We showed that irreversible BTKis broadly prevented IgE-mediated degranulation and cytokine production in primary human mast cells and blocked allergen-induced contraction of isolated human bronchi. To address their efficacy in vivo, we created and used what we believe to be a novel humanized mouse model of anaphylaxis that does not require marrow ablation or human tissue implantation. After a single intravenous injection of human CD34+ cells, NSG-SGM3 mice supported the population of mature human tissue-resident mast cells and basophils. These mice showed excellent responses during passive systemic anaphylaxis using human IgE to selectively evoke human mast cell and basophil activation, and response severity was controllable by alteration of the amount of allergen used for challenge. Remarkably, pretreatment with just 2 oral doses of the BTKi acalabrutinib completely prevented moderate IgE-mediated anaphylaxis in these mice and also significantly protected against death during severe anaphylaxis. Our data suggest that BTKis may be able to prevent anaphylaxis in humans by inhibiting FcεRI-mediated signaling.
Keywords: Allergy; Immunology; Mast cells; Protein kinases.
Conflict of interest statement
Figures




References
-
- Kuehn HS, Swindle EJ, Kim MS, Beaven MA, Metcalfe DD, Gilfillan AM. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells. J Immunol. 2008;181(11):7706–7712. doi: 10.4049/jimmunol.181.11.7706. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

